Burning in pure oxygen
Pure oxygen kindles a smoldering splint!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Keep a bowl of water nearby during the experiment.
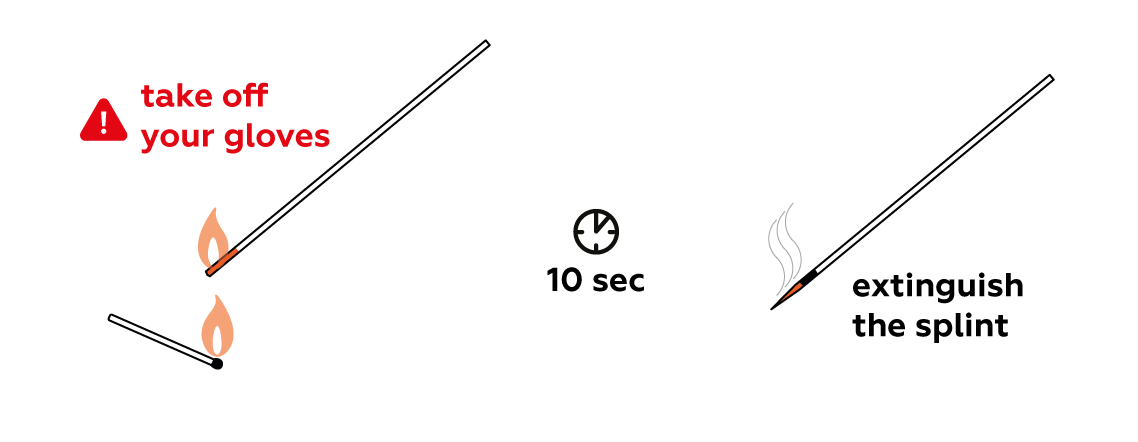
- Remove protective gloves before lighting the splint.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
You can purchase hydrogen peroxide at your local drugstore. Look for concentrations ranging from 3–5%.
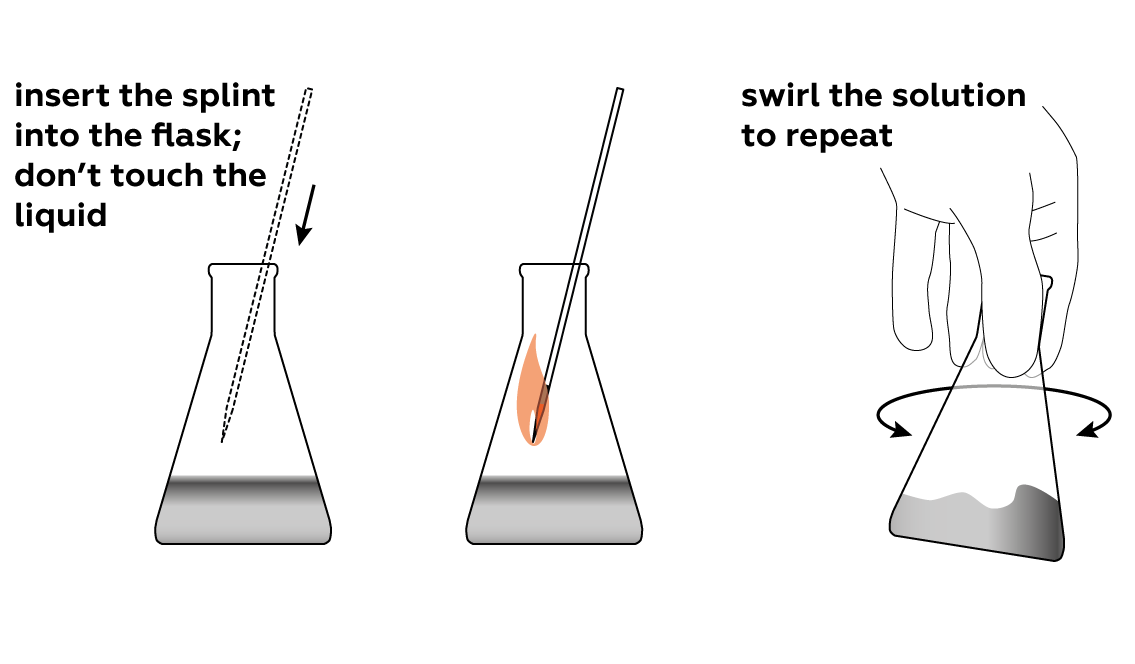
Make sure that the splint is still smoldering when you lower it into the flask, and don’t let it touch the solution.
Step-by-step instructions
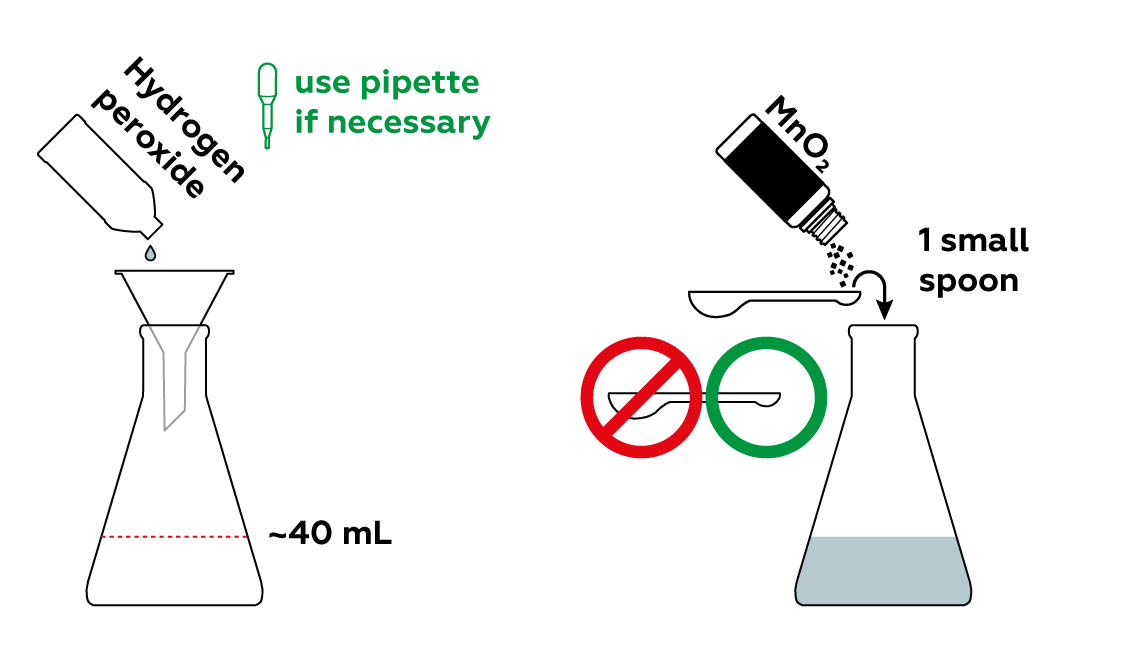
Add some manganese dioxide MnO2 to a hydrogen peroxide H2O2 solution to produce oxygen O2.
Make a splint smolder.
See what happens when a smoldering splint meets pure oxygen.
Disposal
Dispose of solid waste along with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description





is rather weak, it will not break on its own. However, this bond breaks easily in the presence of a catalyst such as manganese dioxide. Very active particles








Why does the smoldering splint ignite?
Smoldering is a slow, flameless form of combustion – the organic substances on the surface of the splint are reacting with the oxygen in the air. Our splint smolders relatively slowly, and over time may cease to burn altogether.
However, there are several ways to increase the reaction rate of the smoldering splint. For example, we can increase the concentrations of the initial reagents. This is exactly why we put the splint into the flask filled with almost pure oxygen – we are surrounding the splint with a much higher concentration of oxygen molecules than can be found in air. This, along with the increase in temperature, dramatically speeds up the reaction rate, so the splint ignites with a bright flame.
Where does the oxygen in the flask come from?
The oxygen is produced via the decomposition of hydrogen peroxide as shown in the following reaction:
2H2O2 → O2 + 2H2O
Why, then, can regular pharmaceutical-grade hydrogen peroxide be stored for long periods of time (up to three years if the bottle remains unopened) without any sign of decomposition? The secret is in the reaction rate. On its own, hydrogen peroxide decomposes very slowly. We can, however, make this reaction proceed faster by using special accelerators known as catalysts. Catalysts significantly speed up reaction rates – sometimes even by a factor of several million!
The antibacterial effect of hydrogen peroxide is rooted in its decomposition. A fresh wound expels drops of blood. Blood, in turn, contains compounds that can accelerate the decomposition of hydrogen peroxide. Thus, upon contact with a wound, hydrogen peroxide decomposes and releases bubbles of oxygen. It’s important to note that this isn’t “regular” oxygen; these are not O2 molecules, but rather very active O∙ particles that eliminate any microbes in their presence. Upon colliding with each other, these same particles bond together to produce molecular oxygen O2:
2O∙ → O2
Why did we add manganese dioxide MnO2?
Manganese dioxide MnO2 serves as a catalyst in the decomposition of hydrogen peroxide. It significantly speeds up the reaction rate. When manganese dioxide is added to regular 3% hydrogen peroxide, the hydrogen peroxide starts to hiss and decompose, releasing a considerable amount of oxygen. Within a short time, oxygen completely fills the flask. This allows us to ignite our splint!