Burning magnesium
The metal behind 19th-century flash photography
Reagents
Safety
- Put on protective eyewear. Conduct the experiment on the plastic tray and in a well-ventilated area.
- Keep a bowl of water nearby during the experiment.
- Keep flammable materials and hair away from flame.
- Avoid looking directly at burning magnesium to prevent eye discomfort.
- Do not attempt to extinguish the solid fuel and magnesium — let them burn down completely. Do not touch the stove after the experiment — wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
It’s best to let it burn out completely. If this is not possible, here are few tips.
First of all, never extinguish magnesium with sand or silica, as this will produce silane SiH4, which is a poisonous gas. Do not use a carbon dioxide extinguisher. Water isn’t optimal either: in large quantities, burning magnesium reacts violently with water, in a reaction resembling an explosion!
In this case, we have just milligrams of burning magnesium, so if you absolutely must extinguish the magnesium fire yourself, use a large volume of water. You can also try cutting off the flame’s oxygen flow. To do so, enclose the burning area securely with a beaker. It’s even better to use a ceramic mug with thick walls.
Try hanging a new strip of magnesium farther from the solid fuel so that only one end of the strip is touching the flame. This should allow the magnesium to burn much more brightly.
Don't worry! Wait for all of the solid fuel to burn out. Replace the old foil with a new piece and try to repeat the experiment using a larger volume of solid fuel. Be sure to position the magnesium so that the flame will only touch one end of it.
Step-by-step instructions
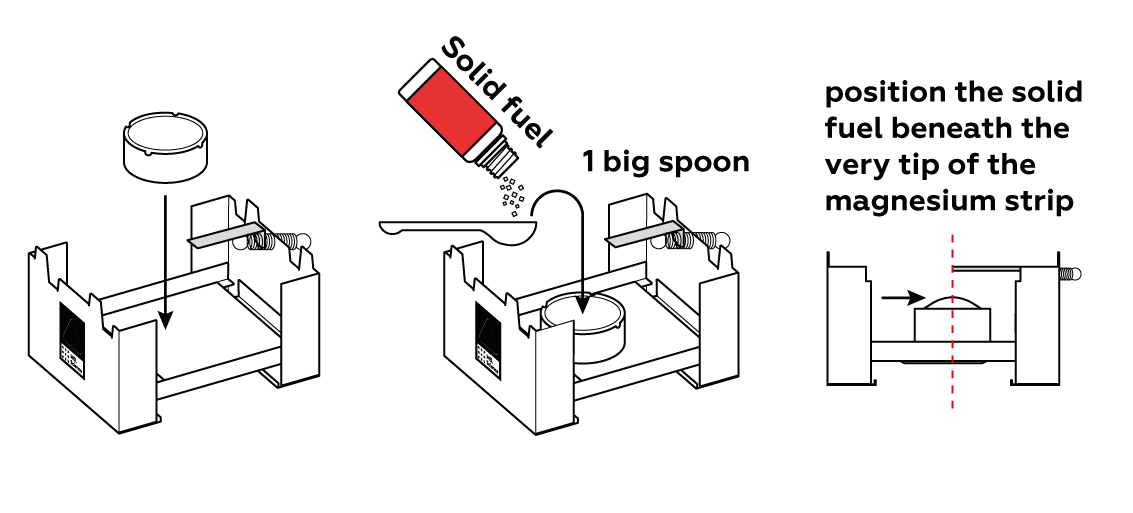
Suspend a piece of magnesium over the solid fuel stove.
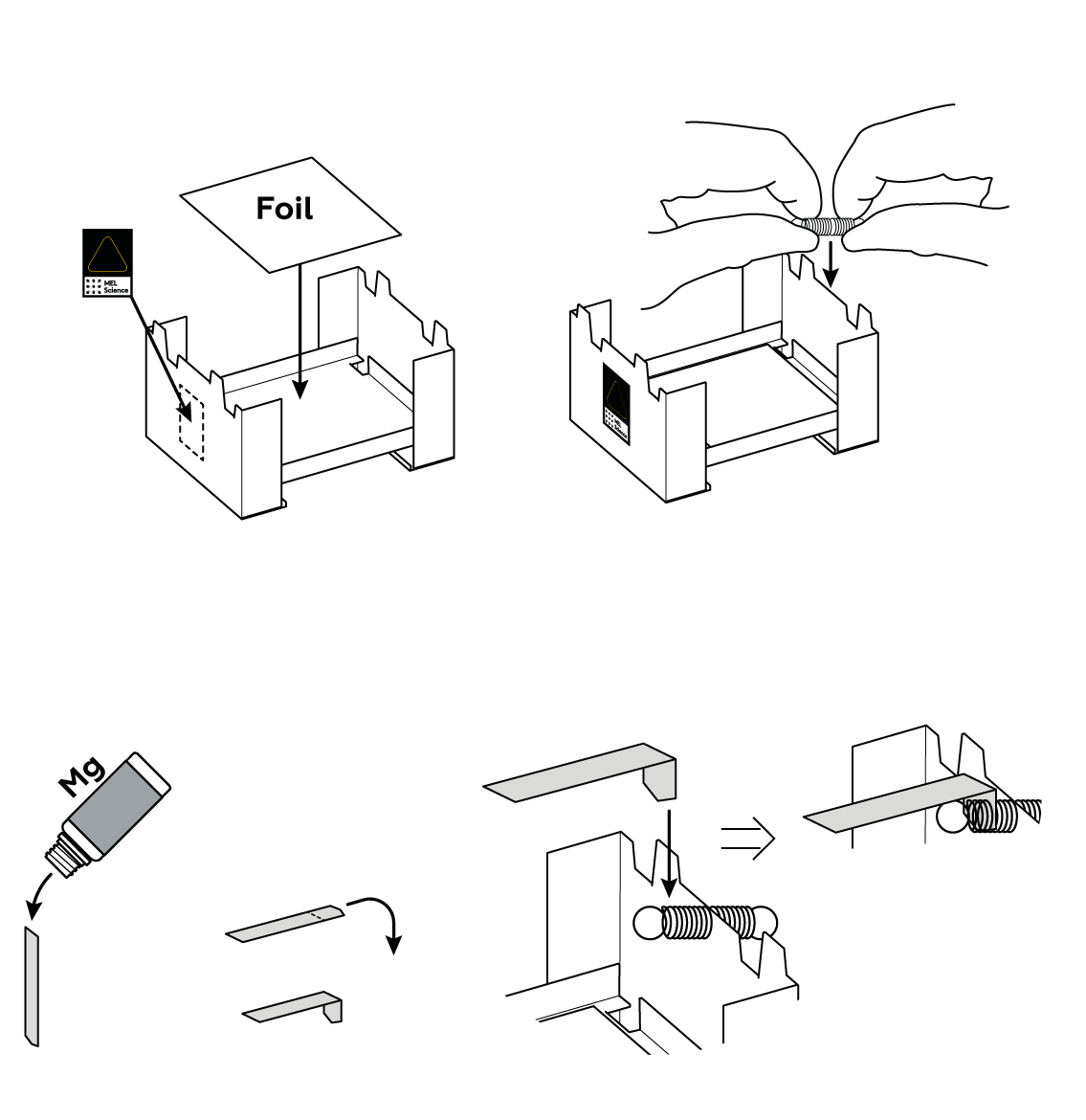
It takes a lot of heat to ignite magnesium. Solid fuel provides just enough.
Get ready! It’s going to get bright!
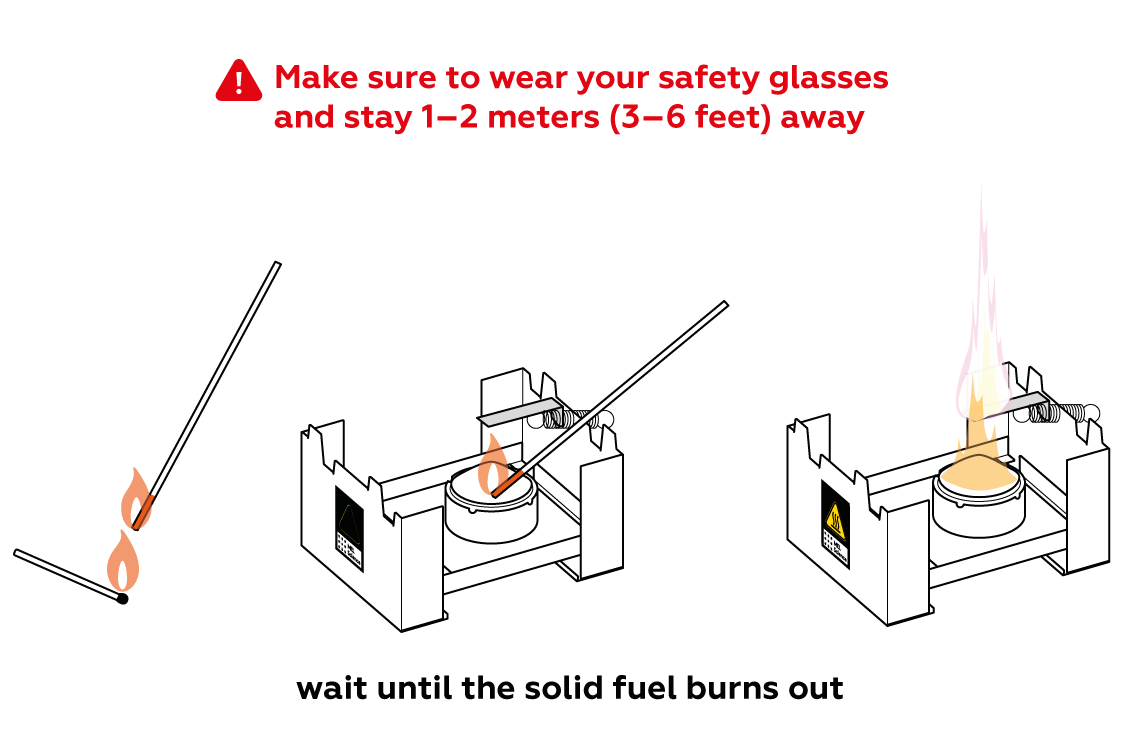
Expected result
Magnesium burns in air very actively, causing a bright glow and releasing a generous amount of energy. The main product of magnesium with oxygen reaction is magnesium oxide MgO.
Disposal
Dispose of the reagents and solid waste together with household garbage.
Scientific description
From a chemistry perspective, burning is the process of giving electrons to an oxidant, usually oxygen O2 

Most familiar metals, such as iron Fe and copper Cu, are in the middle of the table 



That’s interesting!
What is magnesium used for?
Magnesium burns extremely brightly, and this property found its use. The spectrum of light released during magnesium burning has a significant ultraviolet component. That's why it was used in photography for a while. Mixtures of magnesium with various oxidizers (barium nitrate Ba(NO3)2, potassium chlorate KClO3 or potassium permanganate KMnO4) were used as a photographic flash because photoplates back then were very sensitive to ultraviolet.
Currently, metallic magnesium is used in signal and illumination flares, fireworks, flash grenades, and tracer bullets as a bright white light source. Mixed with solid oxidizers, metallic magnesium may also serve as rocket fuel.
Magnesium is widely used in industry not only due to its flammable properties. Thanks to its lightness, magnesium-based alloys found their use in aircraft and rocket mechanical engineering: for instance, in airplane chassis production. Adding small amounts of lanthanum (La) and cerium (Ce) to the alloy make it suitable for high-temperature applications–for example, in aircraft engines. Magnesium oxide MgO, with the addition of magnesium chloride MgCl2 (20%), is the main component for a magnesite cement, a hard fire-proofing material.
Magnesium-based electrochemical power sources are used as a mission-critical energy supply. Such power sources exhibit a high level of self-discharge, hence, their assembly should be performed immediately prior to use. However, as a benefit, they provide more electric power (or amperage) in comparison with most regular electrochemical power sources. Moreover, magnesium-sulfur batteries are in development currently. They may replace today’s lithium-ion batteries in the near future.
