Atom size trends
The best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript
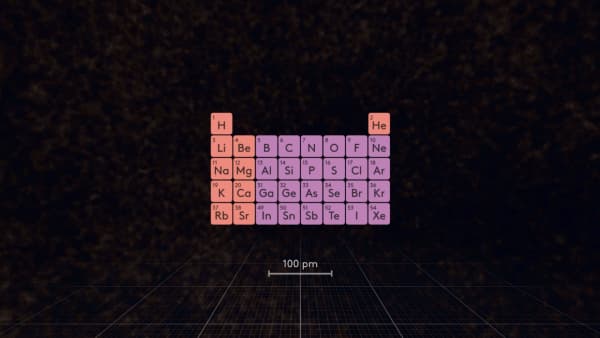
Recently we talked about how to determine the size of an atom. Today, we will look at the patterns of change in the size of atoms in the periodic table.
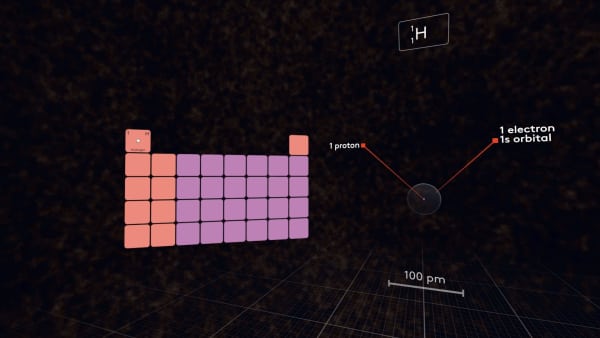
First, let's look at the hydrogen atom.
It has one proton in the nucleus and one electron in the 1s-orbital. It is the first atom in the periodic table.
To make the next atom we should add an electron, a proton, and two neutrons to the hydrogen atom.
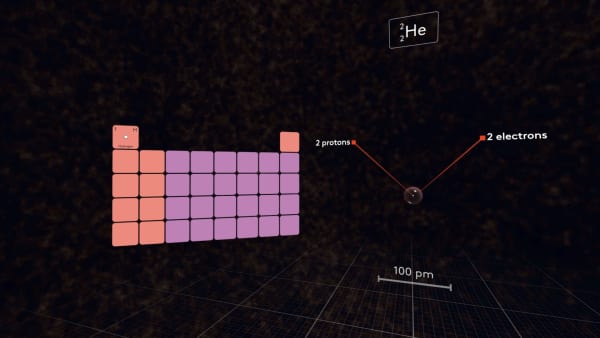
Add an electron.
It occupies the same orbital as the first electron, but the size of the atom becomes much larger.
In the nucleus, there is only one proton, and both electrons feel its attraction, but two electrons in a small 1S orbital also strongly repel each other, so they need much more space.
Add a proton. Now the nucleus attracts electrons more strongly and the size of the atom has decreased. Add two neutrons and put the helium atom next to the hydrogen atom.
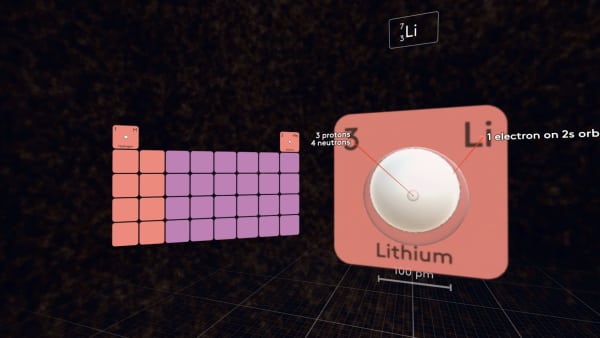
To make the next atom, again we need to add an electron, a proton, and two neutrons. Add a proton.
The size of the atom decreases. Add an electron. The atom increases in size! But why?
The third electron occupies the 2s-orbital, which is much bigger than the 1s-orbital.
Add the neutrons and put lithium in its place in the table.
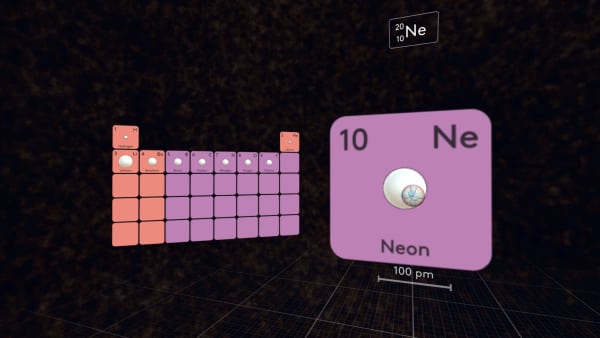
Every subsequent element in the second period has one more proton than the preceding element, so the nuclear charge increases and the outermost electrons come closer to the nucleus.
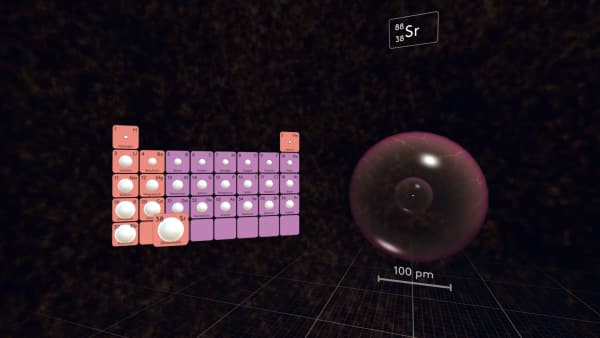
In the third period, the first atom will again be very large and the size of the atoms will decrease along the period.
So atomic size generally decreases as you move from left to right across a period. And atomic size increases as you move from top to bottom through a group.
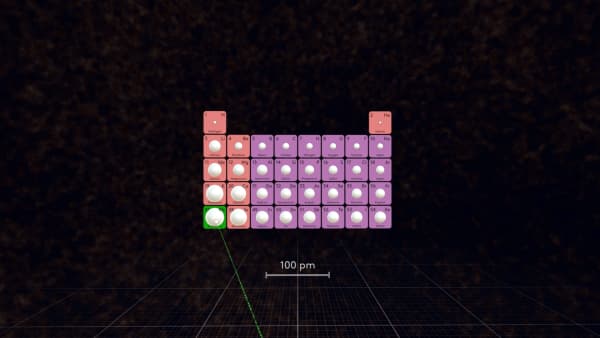
Look at the periodic table and determine: which atom is the largest in this table?
In the first 5 periods, Rubidium is the largest atom.
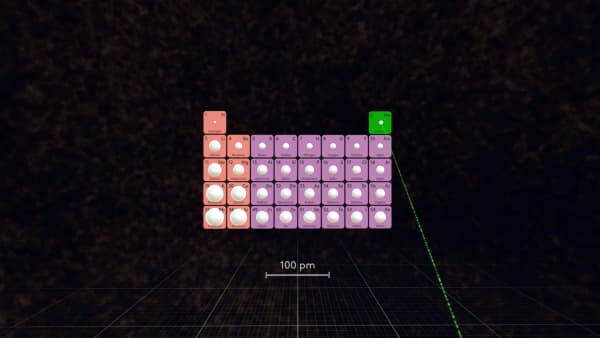
Which atom is the smallest in the periodic table?
That’s right, but remember that helium is a noble gas which does not react with other atoms, so among atoms that can form substances, fluorine is the smallest.
Teacher's notes
Keywords
atoms, atomic structure, electrons, protons, atom size, atomic radius, coulombic force
Students will
- Revise the elements that are arranged in the Periodic Table according to their atomic number: number of protons (equal to the number of electrons).
- Learn that protons attract electrons, but electrons themselves repel.
- Learn that atomic size decreases through the periods.
- Learn that atomic size increases through the group.
- Find the largest and the smallest atoms in the table.
History and sources of knowledge
- Experiments to measure the atom size: Rayleigh experiment with oil.
- Modern methods: x-ray and spectroscopy.
Topics to discuss
- Atom sizes: definition, problems of measurement. (Revise VR lesson ’Atom size’)
- Counterintuitive trends in periods: the atoms became heavier but atomic size decreases.
- Forces determining the size of atoms.
- Periodicity in atomic size.
Calculating
- Why the atom size decreases in periods?
- Why the atom size increases in groups?