Atoms in gases
You will zoom inside a helium balloon and see that helium gas consists of atoms that are flying. You will see that atoms in gas are further away from each other than in solids. Finally, you will see how atoms start flying faster if you increase the temperature.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript
In our previous lesson you saw that solids consist of small atoms that are vibrating. Now, what about gases?
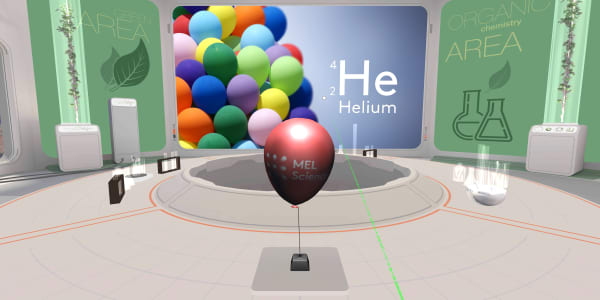
We have a helium-filled balloon in our lab. Certainly you've seen balloons like it at birthday parties.
Let's look inside. Ready to dive?

We have to zoom in a billion times to see the individual atoms.
You can see that helium gas is made of small helium atoms, but these atoms are much further apart than the ones we saw in a diamond.
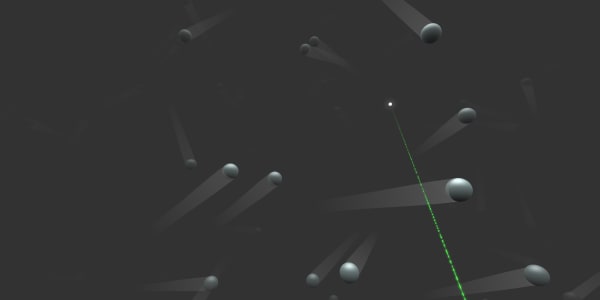
In real life the atoms don't stay still. Remember, in solids they vibrate. Let's switch time on and see what happens in a gas. Ready, steady, go … .
They're flying!
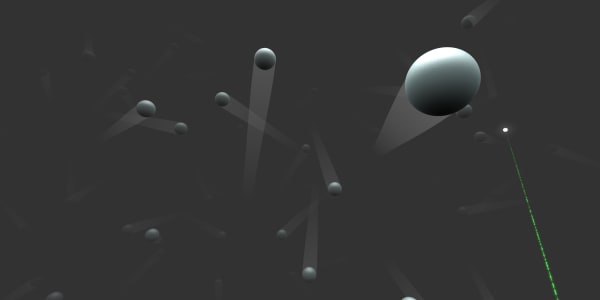
This gas is at normal room temperature, but what will happen if we increase the temperature?
Let's heat the gas to 1000 degrees.
You see our atoms are flying much faster. Actually, such random atom movement is what we call temperature. The faster atoms fly or vibrate, the higher the temperature will be.
Take a few moments and fly around inside.
You're now as small as an atom. The next time you're at a birthday party and you see a helium balloon, you can tell your friends that you flew inside the same kind of balloon and saw these tiny atoms.
Remember that, in the previous lesson, you saw that in solids the atoms are close together. However, in gases there is plenty of space between atoms. That's why gases are much lighter than solids: There are far fewer atoms in the same volume.
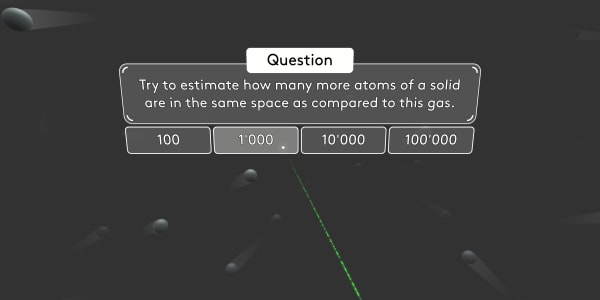
Try to estimate how many more atoms of a solid are in the same space as compared to this gas.
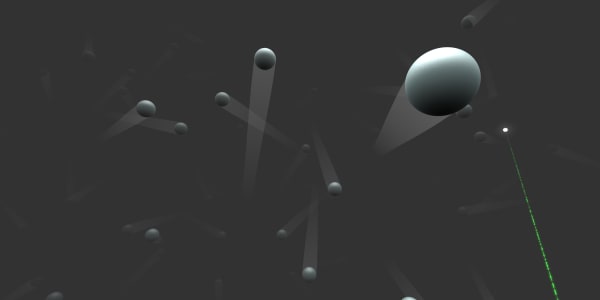
There are about a thousand times more atoms in the same space in solids compared to gases. That is why gases are, on average, about a thousand times lighter than solids.

Let's go back to our laboratory.
Now we have a tricky question for you. Are there things that do not consist of atoms?

Not everything consists of atoms. Light, for example, consists of photons, which are different kinds of particles with different properties.
Teacher's notes
Keywords
atoms, matter, state of matter, gas, temperature
Common misconceptions
- Gases are not matter because we can't see them or feel their mass.
Students will
- Learn that atoms in gases are much further away from each other than in solids
- Find out that atoms in gases are flying freely
- See that when the temperature increases atoms in gas start to move faster and when the temperature decreases they slow down
- Be able to compare the density of gaseous objects to solid ones
Hands-on activities
Before VR
The goal is to demonstrate how much denser liquid and solid objects are compared to gases. Ask students to determine (make measurements with a set of weights and then count) how many helium balloons a student needs to lift him/her up in the air.
Equipment: balloons filled with helium, set of weights.
After VR
Syringe experiment.
The goal is to show students how powerful gas particle motion is. Ask students to pull out a syringe plunger and keep it in place with their fingers. Let them see how easy they can do it. Then ask them to close the hole with their finger and pull out the plunger again. Let them feel that it becomes much harder to hold the plunger so that they can see how rigorously gas particles move and hit everything around.
History and sources of knowledge
- The ancient Greek theory that air was one of the basic elements.
- Discovery of different gases in the air.
- Our knowledge about air as a mixture of gases.
- Particle motion in gases: under a microscope, we can see the Brownian motion of tiny smoke particles in the air. It proves that air consists of tiny particles moving chaotically.
Topics to discuss
- How do we know that there are gas particles around us if we can't see them?
- The speed of gas particles.
- How much denser are solids than gases?
- Billions of gas particles are hitting us, why don't we feel it?
Fun facts and quotes
- If you stretch out the palm of your hand, you actually hold about 100 kg of air.
- The heaviest gas is sulphur hexafluoride. If you pour this gas in the tank a tin foil boat would float on top of it.
- Air is roughly 1,000 times less dense than water. 1 cubic meter of water weighs 1 ton or 1,000 kg, while 1 cubic meter of air weighs approximately 1.3 kg.
Questions
- Why can gases change their shape and solids can't?
- Why can gases change their volume and solids can't?
- What is denser gas or solid and why?
Calculating
In China, one of the world’s fastest trains can reach a speed of 430 km/h (approximately 0.2 km/s). In the air, oxygen particles travel at around 500 m/s. Which is faster oxygen particles or the train?