Copper mirror
Copper on the bottle's walls, who's the fairest of them all?
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Of course! An equally impressive silver mirror can be obtained relatively simply. Watch this video to learn how!
Step-by-step instructions
Prepare a solution of sodium acetate CH3COONa and sodium ascorbate in a bottle.
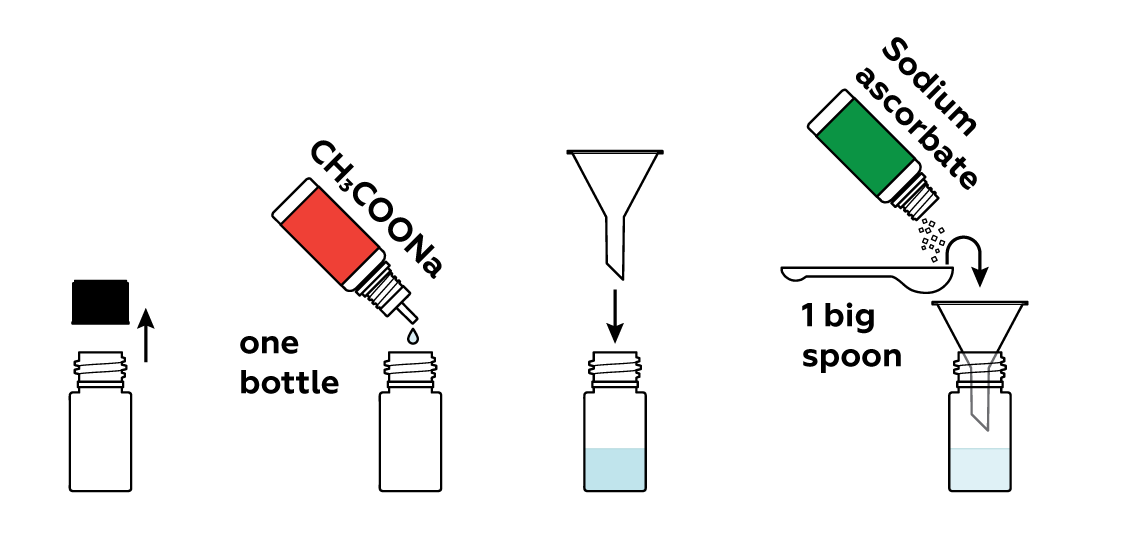
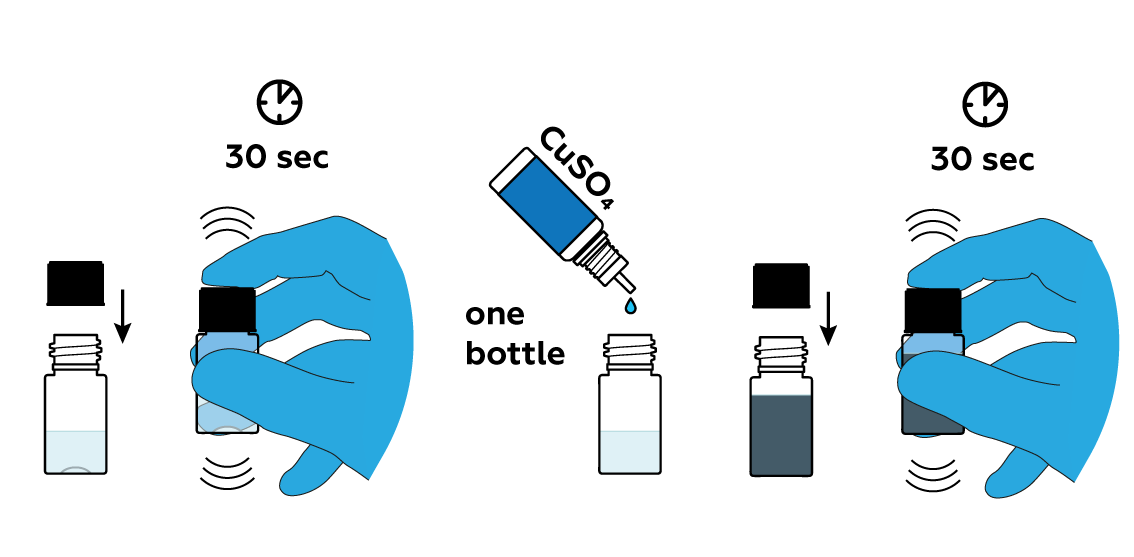
Add some copper sulfate CuSO4 solution.
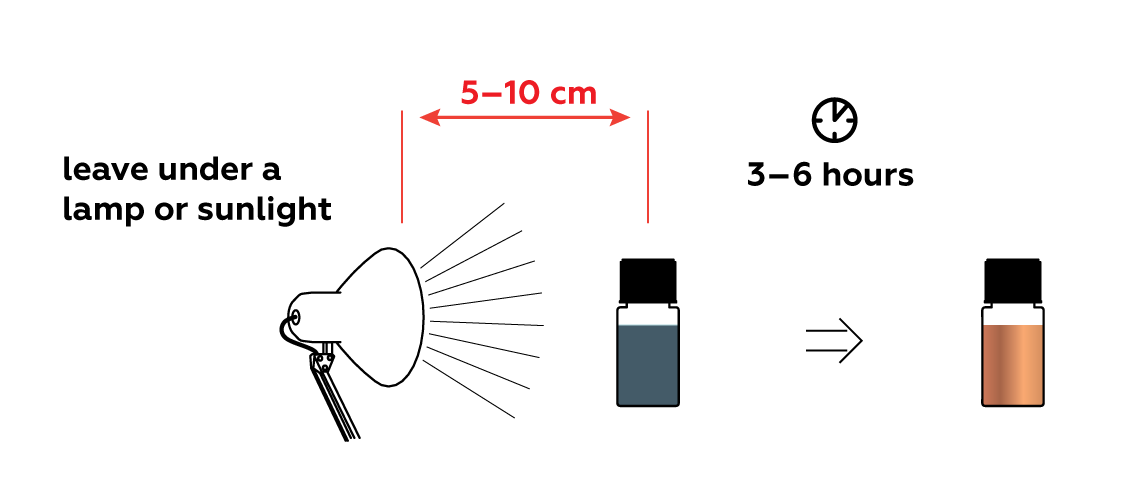
Expose the mixture to light to speed up the reaction. Observe the result after several hours—the inside of your bottle is covered with a reflective layer of copper!
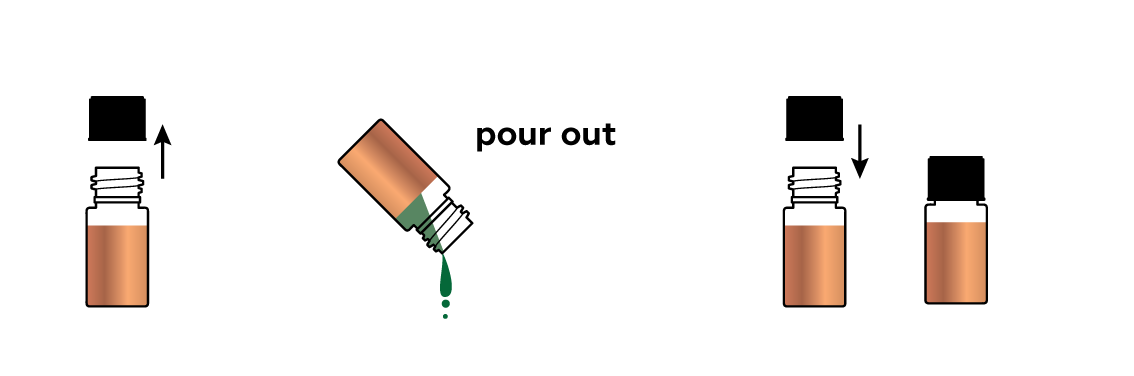
Pour out the contents of the bottle.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
It may seem that metallic copper materialized on the inside of the bottle out of nowhere. In reality, it was there all along. The CuSO4 solution you added contained Cu2+ ions 




That’s interesting!
The history of mirrors
The history of mirrors is just barely shorter than the history of humankind. Nature first inspired us to look at our own reflections – the first “mirrors” were the surfaces of stagnant bodies of water such as lakes or ponds. The first man-made mirrors consisted of either polished stone or black obsidian (volcanic glass) and can be dated to 6000 BC. More mobile metallic mirrors appeared in the Bronze Age (3500-1200 BC), when people learned how to brighten pieces of bronze and turn them into thin plates. However, such surfaces quickly oxidized and dulled; they required polishing almost daily.
The advent of glassblowing opened the door to an entirely new approach to mirror-making. Molten tin was poured into a glass sphere, evenly distributed over the entire surface, and allowed to cool. The glass was then shattered, leaving the reflective tin coating behind. The concave surface distorted the image, but this drawback was eliminated when craftsmen learned how to make flat panes of glass.
The next step forward began with the introduction of an alloy of mercury, or amalgam, to the process. Instead of liquid tin, foil was coated with a layer of mercury and covered with a glass plane. However, this method was detrimental to health due to the high toxicity of mercury vapors. Later, mercury was replaced by silver, resulting in a shiny surface with excellent reflective characteristics. Today, mirrors are more frequently produced by depositing much cheaper aluminum directly onto glass.