Glowing matter
Make a glowing substance from scratch!
Safety
- Do not aim UV light at eyes or face.
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Remove protective gloves before lighting the splint.
- Keep flammable materials and hair away from the setup.
- Do not touch the stove after the experiment. Wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
Step-by-step instructions
Neither citric acid nor urea are particularly luminous on their own. Both look plain and white under normal light. Even if you illuminate them with your ultraviolet flashlight, they’ll still look pretty normal. If you simply mix these two substances in a~dish, not much is going to happen.

To make urea react with citric acid, you need to melt the two substances. To do this, heat the mixture gently with a candle.
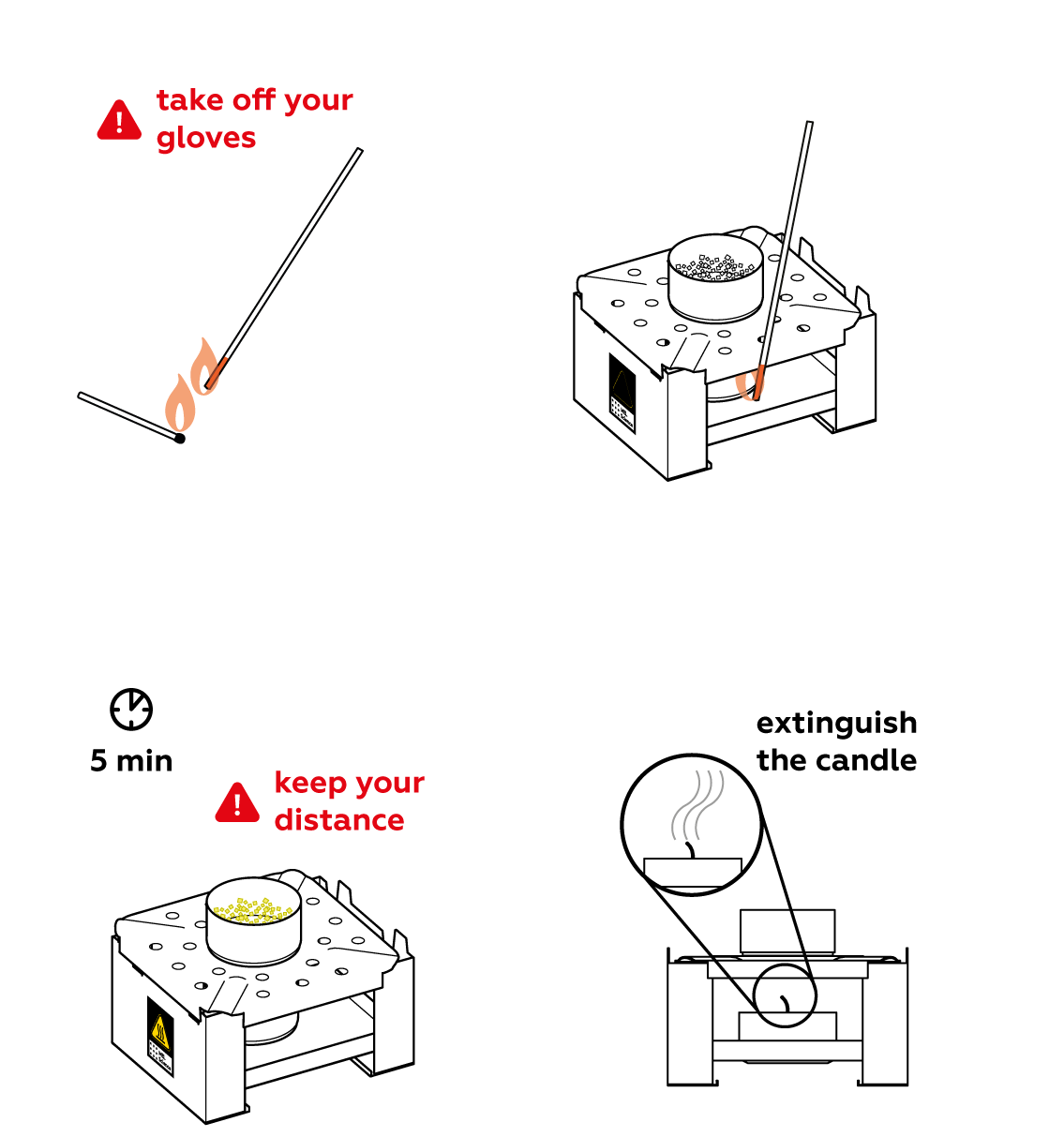
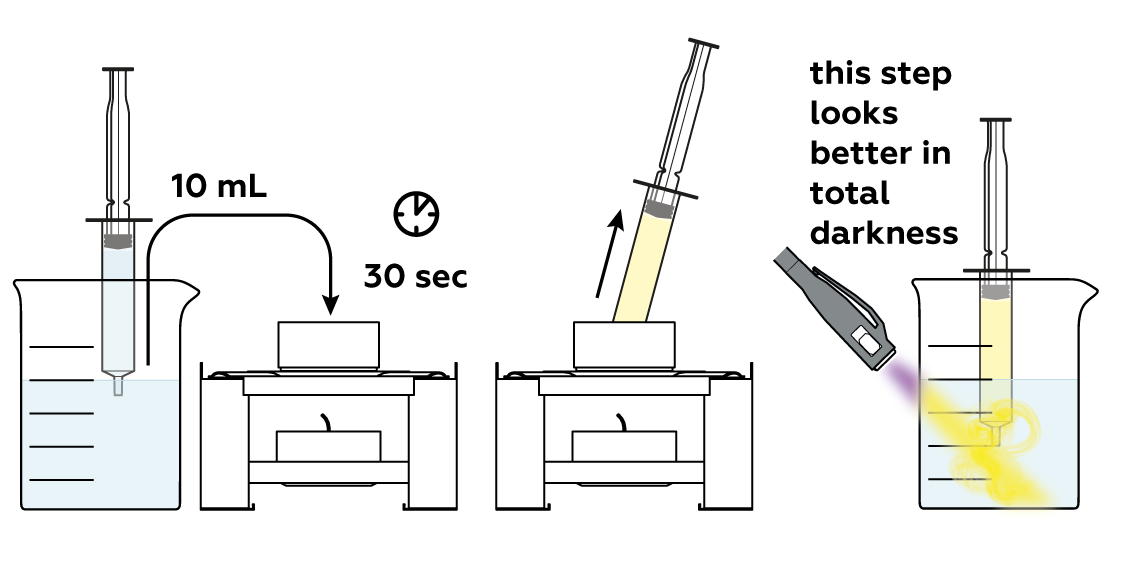
When both substances are melted, their molecules start mixing and mashing to produce several new compounds. Dilute the substance with water and illuminate it with the UV light.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
This compound is only clearly visible under UV light—if it weren't for how weakly it glows, this would make it a passable invisible ink. Colorless compounds with a stronger glow are much more difficult to synthesize, but you've actually got one in the markerthat comes with your UV light!
UV light isn't the only light that can make things glow—some substances can emit light after exposure to visible light. Some substances can even "accrue" light energy and release it slowly over a long period of time. There's a paper in this set that's treated with such a substance — have fun playing with it!
That’s interesting!
Glowing in UV light is more useful than you'd think!
The substance you prepared is similar to luminescent dyes, which are commonly used in decorations due to their ability to glow different colors in total darkness when under UV light. But their abilities don't end there!
UV-luminescent marks are often applied to banknotes. Despite being invisible to the naked eye, they allow cashiers and bank tellers to easily detect counterfeit money using UV light (counterfeit banknotes often don't have this feature). Chemists use UV light in chromatography to detect if substances that are invisible in normal light have separated.
Mercury's ability to emit strong UV light is used in high-pressure mercury vapor street lamps and home compact fluorescent lamps (CFL). In both types of lights, UV is converted to safe white light using phosphor. Nowadays, mercury lamps are being replaced with white LED lights, but white LED light is obtained using the same principle. Ordinarily, common white LEDs use a blue chip and a substance that glows red or yellow in the blue light. But there are also LEDs that use UV light instead of blue light to generate a more diverse spectrum of light that is more pleasant to the eyes.
Your box contains a 405 nm flashlight, which can make a lot of materials glow. But if you're lucky enough to obtain a 365 nm flashlight, you can embark on an incredible nocturnal adventure. Many flowers, flies, and bugs glow in the dark if you illuminate them with a beam of this wavelength! In addition to just being cool, this can help you avoid dangerous spiders or scorpions.