Acid switch
Turn luminescence on and off at will!
Safety
- Do not aim UV light at eyes or face.
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
Step-by-step instructions
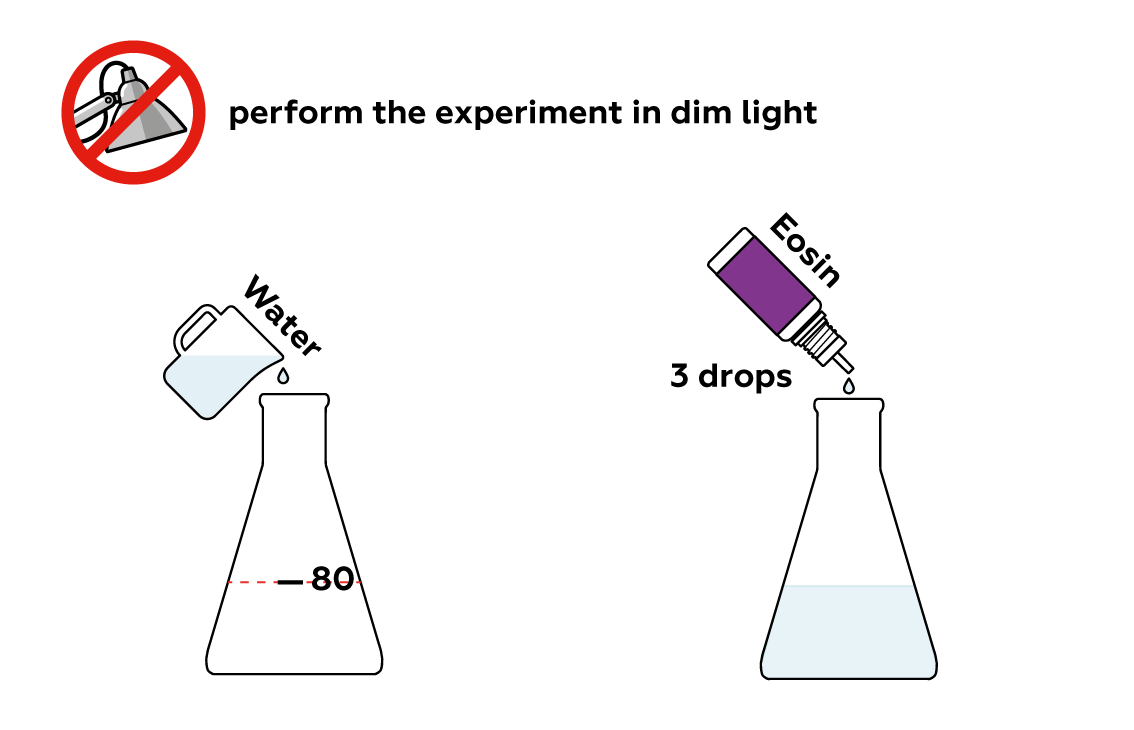
Eosin is a~dye with an intense color. Even a few drops can turn a~considerable quantity of water red.
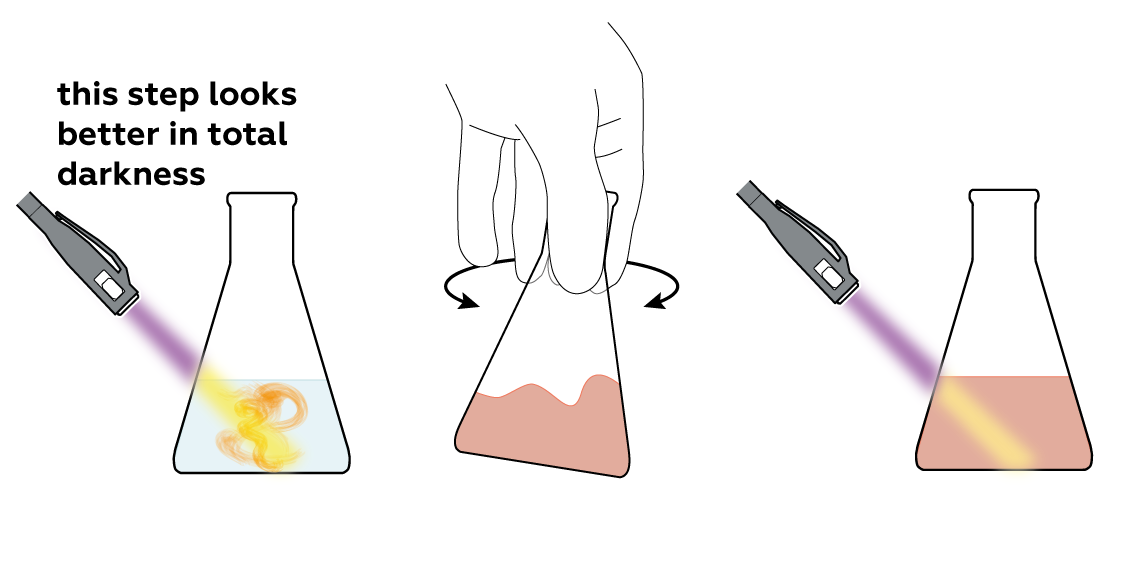
However, if you shine a UV light on it, it starts glowing yellow-green!
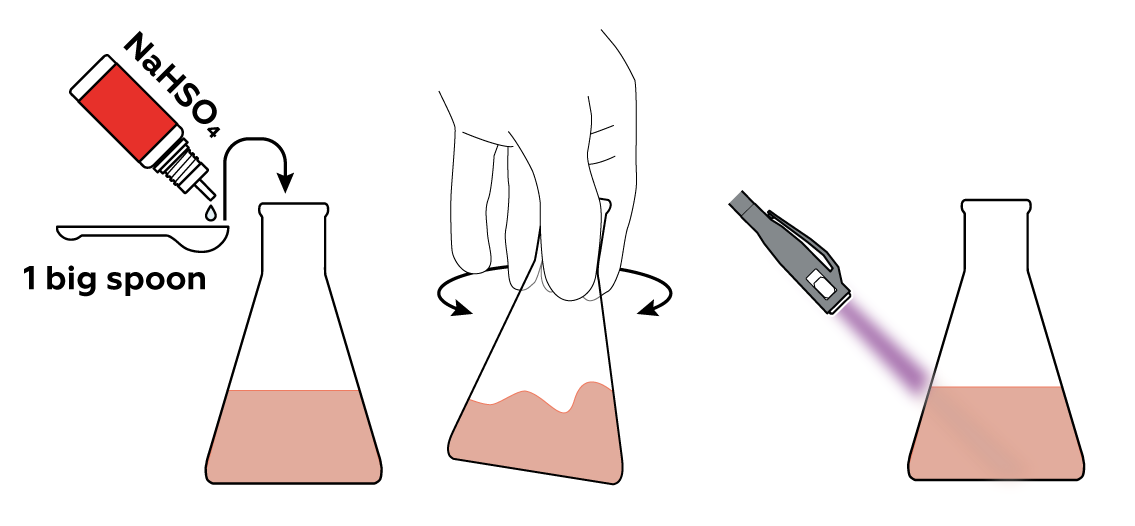
When you add NaHSO4 to your eosin solution, nothing seems to change: it's the same orange-red liquid. However, watch what happens if you shine the UV light on it now. The yellow-green glow has disappeared! That's because the H+ from NaHSO4 binds with eosin, making it lose the ability to glow like this.
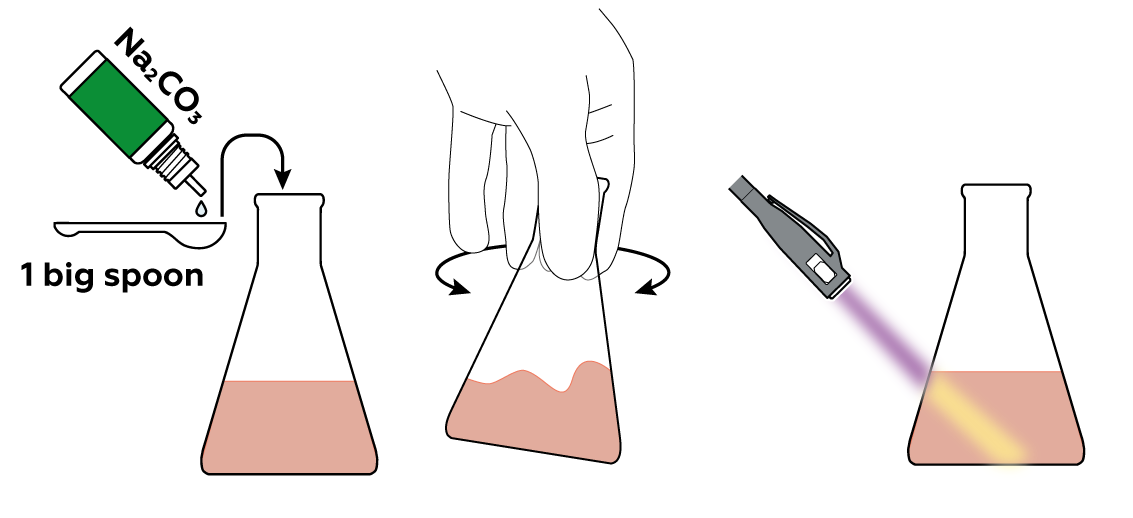
If you think you've ruined your cute glow-in-the-dark flask, no worries! Just add Na2CO3 and watch the glow return. That's because CO32- from Na2CO3 rips H+ from eosin, restoring its ability to glow under UV light.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
In this experiment, NaHSO4 acts as an acid, because it yields H+ ions in water. Na2CO3, on the other hand, acts as a base, because it can catch stray H+ ions and even react with water H2O to yield OH- ions. As you've seen, when there's a lot of H+ ions out there, eosin loses its ability to glow under UV light. In other words, eosin doesn't glow in an acidic environment, but glows in a neutral or basic environment. In fact, you can "turn" the glow "on" and "off" using NaHSO4 and Na2CO3 quite a few times. This technically makes eosin a pH indicator!
Curiously, eosin enjoys its greatest prominence in the field of biology. Eosin is good not only at being red and glowing under UV light, but also at binding to proteins. This combination of properties makes it perfect for staining some biological samples. You see, biological samples are rarely easy to distinguish under a microscope. More often than not, it's nearly impossible to discern what is where—everything is more or less the same color. But allowing red eosin to bind to protein-rich microscopic structures makes them clearly visible.
That’s interesting!
What else is eosin useful for?
Before moving on to other applications, let's talk a bit more about why eosin is used in microbiology.
Most of a cell's contents and subunits, called organelles, are white and almost transparent (with the exception of chloroplasts and a few others). This means that tinting the different cell components different colors is a crucial part of the examination process. Scientists found that some dyes, like hematoxylin, bond effectively to the cell nucleus. Others, like eosin, bond to other cell parts. Working in tandem, these dyes make an effective team to tint tissue samples. (Note that the dyes must be different colors.) Blue hematoxylin and pink eosin work perfectly together; this pairing is called an H&E stain. That’s why, if you happen upon a micro cell photo, it will often be pink and blue.
Eosin is also used in analytical chemistry. A reaction similar to the one you conducted is sometimes used to determine the concentration of an acid or a base. It also comes in handy when detecting Вr-, I-, and SCN- ions.
Eosin was discovered more than 150 years ago, attracting attention with its bright, fluorescent pink color. It was used to make red inks, wax pencils, and even lipstick, until it was replaced with safer natural compounds.
Some painters also used eosin as a dye, the most famous being Vincent van Gogh. Unfortunately, as eosin degrades over time due to radiation from the Sun, some of the red sections of van Gogh’s paintings have gradually turned brown.