Magic liquid
pHantastic colors and how to get them!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the protective underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The colors of the solutions depend on their pH, and their exact pH values can be influenced by various factors, such as the temperature or the concentration of reagents in the solution. Therefore, the solutions can have many different shades of color. You can expand on the experiment by making the solutions in the cups more saturated (adding more reagent) or increasing/decreasing the water temperature and observing how the color of the liquid changes. Also, try using clear, bottled, non-carbonated water. Tap water, carbonated water, or water with any sort of additives often has a non-neutral pH.
Step-by-step instructions
You can picture water molecules H2O as consisting of two parts: H+ and OH-ions. These parts do exist in real life, but once they meet, they form water H2O. A pH indicator, such as thymol blue, shows if there’s an excess of either component in a given solution. If thymol blue in a solution is yellow, then the H+ and OH- ions in that solution are balanced.
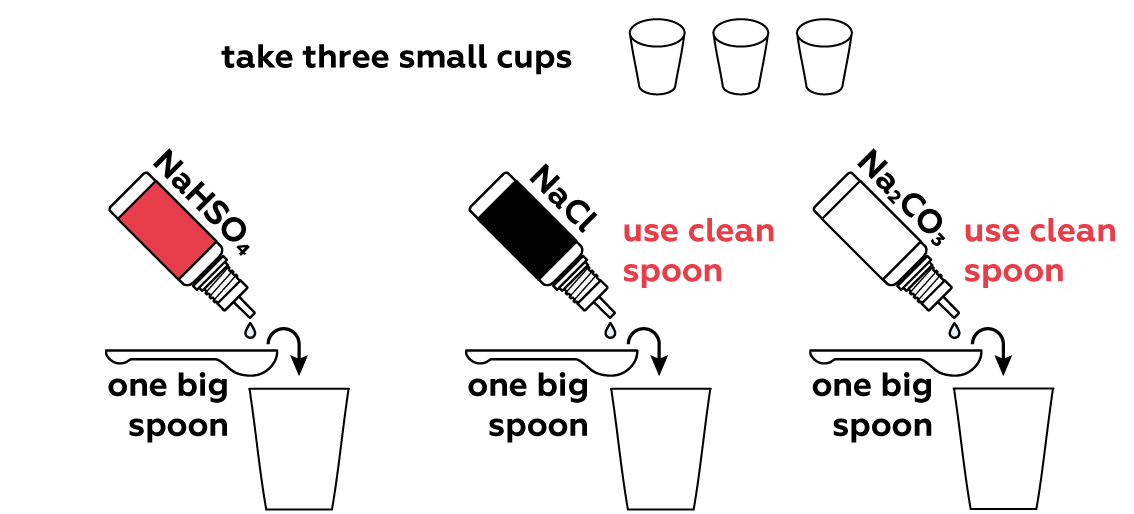
Many compounds can either add H+ or OH- ions to a solution directly, or rip H+ or OH- from water molecules H2O. This shifts the H+\OH- balance of the solution. Let's see if any of your three sample compounds can do this.
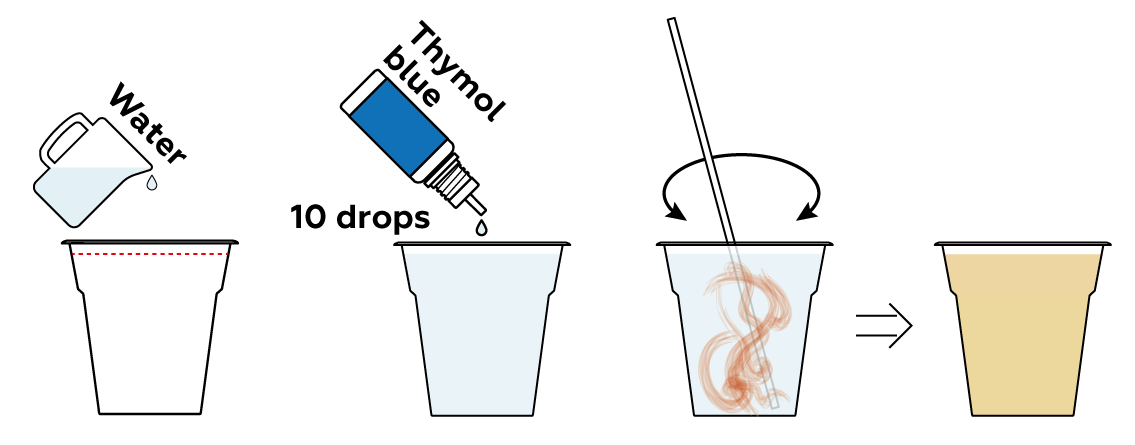
Add some of your pH indicator solution to your samples and see what happens.
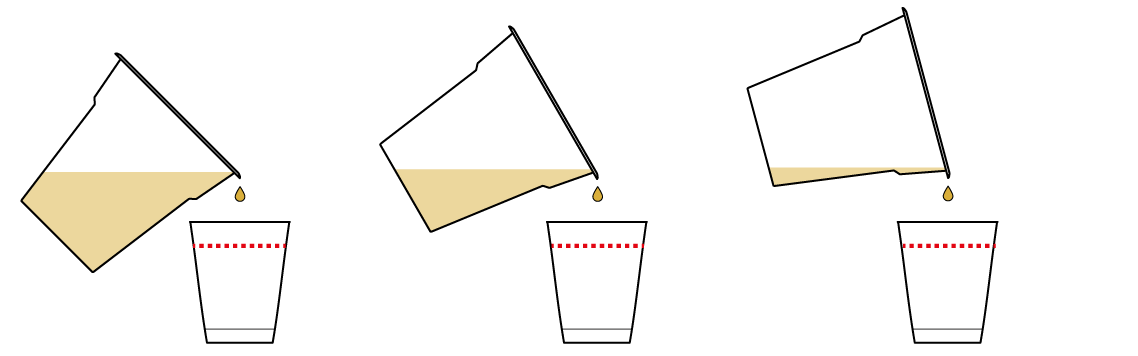
Whoa! It's a color-changing situation!
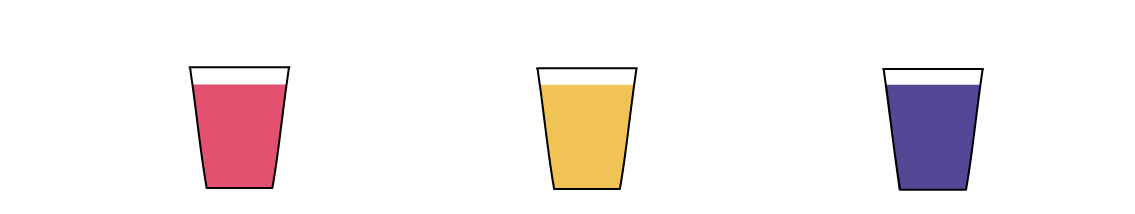
The cup with NaHSO4 turned red. This means that NaHSO4 caused an excess of H+ ions in the solution. In fact, the culprit is the same "H" that’s in the middle of the NaHSO4 formula!
The cup with NaCl didn't cause the thymol blue solution to change colors. That's because it contributed neither H+, nor OH- to the solution, leaving the balance intact.
The cup with Na2CO3 turned blue. This means that Na2CO3 caused an excess of OH- ions in the solution. How did this happen? The CO32- part of Na2CO3 tends to bind with H+ quite strongly. So much so that it can even rip it away from water H2O molecules, leaving an excess of OH- ions behind.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
To define exactly how great the H+ 
imbalance is, chemists use a scale called "pH." Pure water


That’s interesting!
Why does the liquid change colors?
To recap: we prepared three disposable plastic cups, each with a small quantity of a different substance, and then added the solution of thymol blue to each of them. Since each cup contained a different substance, the molecules of thymol blue found themselves in quite diverse neighborhoods and therefore behaved differently in each.
Let us analyze in detail what happened in each cup. We will refer to the thymol blue as “Ind” (Indicator).
Cup #1 (red)
We put 1 big spoon of sodium hydrogen sulfate NaHSO4 solution in the first cup. When dissolved in water, it dissociates cheerfully into three charged particles (called ions):
NaHSO4 → Na+ + H+ + SO42-
As a result, many H+ particles (hydrogen ions or protons) are released into the solution in the cup. Due to the large quantity of protons H+, the acidity of the medium increases and the thymol blue (Ind2- – blue) turns red H2Ind.
Cup 2 (yellow)
In the second cup, all the thymol blue turns into yellow HInd-. In fact, the medium in the third cup is almost neutral. This happens because sodium chloride dissociates completely into Na+ and Cl- ions, and as you can see, there are no protons H+ or hydroxyl OH- ions to make the medium basic or acidic.
NaCl → Na+ + Cl-
Cup #3 (blue)
In the last cup, thymol blue is present in the form from which it derives its name – blue, indicating that the medium in the cup is basic. This is the result of water’s interaction with sodium carbonate Na2CO3, which yields the OH- ions responsible for the basic medium. The indicator thus becomes Ind2- :
Na2CO3 + H2O → 2Na+ + HCO3- + OH-
