Disappearing iodine
Disodium disulfite, a reducing agent, removes iodine stains


Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The iodine stain did not disappear. How can I fix it?
Try to add about 5-10 drops of Na2S2O5 solution onto the stain. Wait a little longer than you did the first time (about 1 minute). Dry the stain with filter paper.
Step-by-step instructions
-
Pour a few drops of iodine 2,5% water solution onto a piece of paper.
-
Take the bottle with 0,5M sodium disulfite (Na2S2O5) solution as a reductant and apply 5-10 drops onto the iodine stain.
-
Wait for 10 seconds.
-
Blot the stain with filter paper. The iodine stain disappears!
-
As a follow up, try to restore the stain: apply 1 drop 2М of hydrochloric acid HCl and 2 drops of 3% Hydrogen peroxide H2O2 solution to an experimental area.
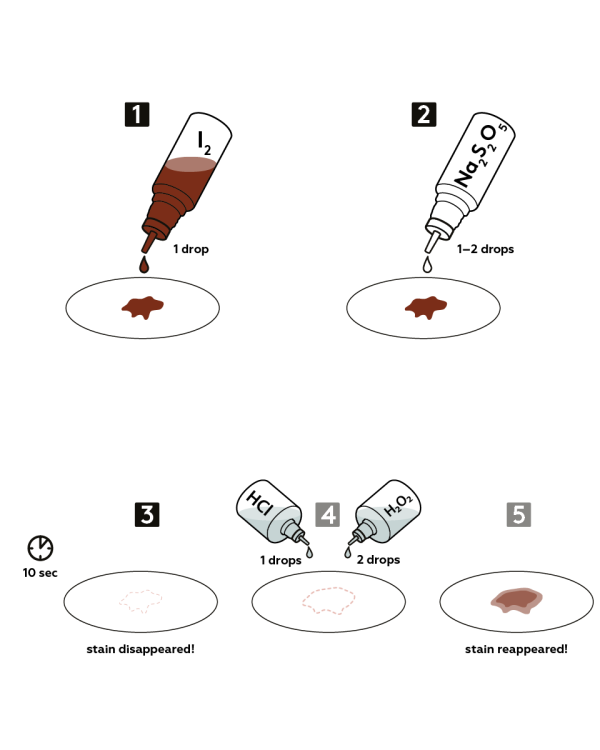
Expected result
The iodine stain fades, as disodium disulfite (Na2S2O5) reduces brown iodine (I2) to colorless iodide ions (I-).
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Why do iodine stains disappear?
We use water solution of disodium disulfite Na2S2O5 to decolorize iodine stain. The following reaction takes place when disulfite interacts with iodine in water:
Na2S2O5 + I2 + H2O → NaHSO4 + HI
Molecular iodine acts as an oxidizer, and disodium disulfite as a reductant.
When iodine I2 disappears from the system, brown-orange color of stains disappears as well.
More details:
This reaction can be divided into two parts: reduction of iodine and oxidation of disulfite:
S2O52– + 3H2O – 4e– → 2SO42– + 6H+
I2 + 2e– → 2I–
If we take into account the number of electrons in the first part, we should multiply the second one by two (to achieve so-called electronic balance):
2I2 + 4e– → 4I–
When we express a reaction like we did it right above, separated into two parts (taking into account electrons, not just molecules or ions), we call these parts half-reactions.
Compare how the reaction equation looks before and after we have applied the half-reaction method:
Na2S2O5 + I2 + H2O → NaHSO4 + HI
Na2S2O5 + 2I2 + 3H2O → 2NaHSO4 + 4HI
What is oxidation?
Redox, or reduction-oxidation, reactions involve electron transfer between two particles (molecules, atoms or ions). An oxidizer (iodine) wants to get some electrons from a reductant (disulfite). Oxidation is an electron transfer process, in which an oxidizer (an element with relative electron deficiency) takes electrons away from an outer electron shell of a reductant (an element with relative electron excess). Therefore, an oxidizer is being reduced (gains electrons) during this process and a reductant oxidized (loses electrons).
More details:
We should introduce another concept to talk about redox processes: the oxidation state of an atom. Oxidation state is a positive or negative number attributed to an element in a compound. This number is a formal charge of an atom in the given compound.
Oxidation state is equal to zero in elementary substances such as iodine I2. When we talk about a one-atom ion (for example, I–, Mn+2, H+, Na+, and K+), oxidation state is equal to the charge of this ion.
The sum of oxidation states of all the atoms in a neutral molecule is always equal to zero. The quantity of atoms should also be taken into account! For example, KMnO4 has a total of 6 atoms: K+, Mn+7, and 4 atoms of O-2. Hence, the sum is: +1 + (+7) + 4*(–2) = 0.
In a compound ion, this sum is equal to its total charge. For instance, HSO4– has H+, S+6, and 4 atoms of O-2, so the sum is: +1 + (+6) + 4*(–2) = –1.
That’s interesting!
Is it possible to remove other stains with disulfite?
First of all, we want to remind you about safety rules. We highly recommend not conducting any experiments with substances that you are unfamiliar with.
Let us analyze which type of stains we can remove with disodium disulfite. In our case, iodine colors the stain. It acts as an oxidizer and is reduced by disulfite to iodine ion I–. The stain becomes colorless. Hence, if we want to remove a stain with disodium disulfite, the stain color should be determined by an oxidizer (such as I2). The oxidizer must be reduced by disulfite to a colorless or highly soluble in water (better both the same time!) form. For example, we can decolorize a purple potassium permanganate (KMnO4) stain or a brown manganese dioxide (MnO2) stain (see «Chemical eraser» experiment). Both of these compounds are reduced to soluble (and usually colorless) manganese Mn2+ salts:
MnO2 + Na2S2O5 + NaHSO4 → MnSO4 + Na2SO4 + H2O
KMnO4 + Na2S2O5 + NaHSO4 → MnSO4 + Na2SO4 + H2O
Does iodine dissolve in water?
Molecular iodine in its pure form is a highly volatile crystalline substance with deep violet color. I2 is poorly soluble in water (approximately 0.3 gram per 1 liter). Therefore, iodine concentration in water is about 0.08%. However, we use 2.5% iodine aqueous solution in our experiment. How is it possible?
It turns out that when we add iodide ions to water (e.g. in form of KI salt), the solubility of iodine increases dramatically. This can be explained by the formation of I3– complex:
I2 + I– → I3–
This complex is highly soluble in water. At the same time, it is not very stable, so iodine is free to participate in reactions as I2. For example, it can be reduced to iodide ion just as it happened in our experiment.