Copper sulfate synthesis
Sodium hydrosulfate reacts with copper


Reagents
Safety
-
Conduct the experiment on the tray.
-
Always wear safety glasses.
-
Keep a bowl of water nearby during the experiment.
-
Do not touch the stove after the experiment. Wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The wire doesn’t turn blue. What’s wrong?
Copper sulfate synthesis from a copper wire isn’t the easiest chemical process. What could possibly go wrong if a compound around the wire didn’t start turning blue within 15–20 minutes?
Effective heating is a key to this reaction. Therefore, first check if the candle is still burning. Perhaps, it isn’t set up right – see the instructions. Carefully examine sodium hydrosulfate NaHSO4 in the beaker: the substance should have melted and be boiling.
Make sure that the wire is immersed in melted NaHSO4. This is very important because the reaction would not proceed without physical contact between copper and sodium hydrosulfate! Use a wooden splint or a black ball-ended rod from another set to carefully move the substance of the wire if needed.
The odors
During the reaction, SO2 gas is released. In our experiment, the amount of this gas released is insignificant to present any danger. Moreover, it even has antiseptic properties. However, avoid sniffing or breathing it in on purpose!
Also, an unpleasant odor may appear when the bottom of the beaker is being covered with too much soot. Don’t worry, it’s all right. Still, it’s best to conduct this experiment in a well-ventilated room.
How to wash candle soot off the beaker?
The bottom of the beaker will probably get covered with soot. It may leave stains, be careful!
When the beaker is cooled down, first clean the soot off with a paper towel or a napkin and then with a damp cloth.
How to remove sodium sulfate from the beaker?
When the beaker is cooled down, fill it up with water. Wait for sodium hydrosulfate to dissolve completely. Now wash the beaker thoroughly with water, several times.
When the glass is cooling down, I hear cracking. What’s happening?
If you hear cracking inside the beaker when it’s cooling down, stay calm: it isn’t going to explode. In fact, it isn’t the beaker that produces that cracking sound: it’s sodium hydrosulfate that is hardening inside it. No action is required – simply let the beaker cool down.
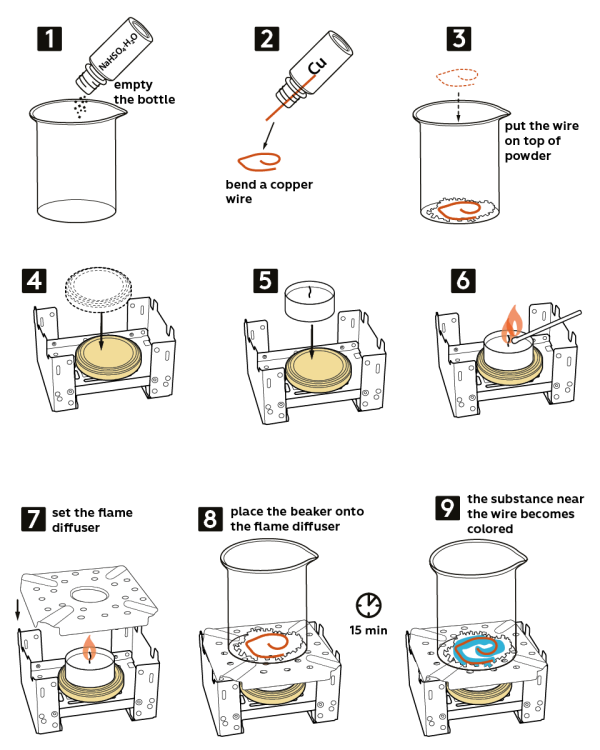
Step-by-step instructions
- Pour sodium hydrosulfate hydrate NaHSO4*H2O (5 g) from the vial into the beaker.
- Take a copper wire from the vial marked “Cu” and bend it.
- Place the bent wire on top of the powder in the beaker.
- Take a stove and place it on a cork hot pad. Place a metal lid on the stove as shown.
- Put a candle on the lid.
- Light the candle.
- Set a flame diffuser on the stove.
- Put the beaker in the center of the flame diffuser. Wait for about 15 minutes. Just in a few minutes, a turquoise blue compound will start forming on the wire.
- The compound formed around the wire is copper sulfate CuSO4.
Expected result
When heated, a mixture of sodium hydrosulfate and metallic copper produces copper sulfate CuSO4 on the wire. The resulting substance colors the NaHSO4 fusion blue-green.
Disposal
Dispose of the experiment residues along with regular household trash.
Scientific description
What happens with a copper wire?
When exposed to sodium hydrosulfate NaHSO4, a copper wire is being dissolved, yielding copper sulfate CuSO4. The latter tints the reaction mixture blue.
Most metals easily dissolve in diluted acids with release of hydrogen. In fact, hydrogen H+ cations (positively charged ions) found in acids composition can withdraw electrons from metals. During this reaction, these cations turn into gaseous hydrogen H2. For instance, such a reaction takes place during zinc oxidation in a diluted acid:
H2SO4(diluted) ↔ 2H+ + SO42-
Zn + 2H+ → Zn2+ + H2↑
However, copper is an unusual metal: it is quite resistant to oxidation. In most cases, copper doesn’t give up its electrons in favor of hydrogen cations, even in a concentrated acid.
Nevertheless, there is another way to oxidize metals – a reaction with particular acids-oxidizers. Among them is well-known sulfuric acid H2SO4, only concentrated. Another one is concentrated nitric acid HNO3. Sulfuric and nitric acids have sulfur S and nitrogen N atoms in their composition. These atoms suffer a significant electron deficiency. That is why they can even take electrons away from “stubborn” copper, thus oxidizing it. In the video below you can see how copper dissolves in concentrated nitric acid, forming copper nitrate Cu(NO3)2 and a brownish gas, nitrogen dioxide NO2. The latter being very soluble in water causes a pressure drop in the vessel and, as a result, a fountain effect.
Naturally, these acids are too dangerous to be included in our experiment sets. Luckily, though, there is a way to synthesize sulfuric acid in situ, i.e. on-site (in our case, right in a reaction vessel).
Indeed, heating sodium hydrosulfate hydrate NaHSO4*H2O yields small amounts of sulfuric acid H2SO4. Further, it can oxidize copper according to the equation below:
Cu + 2H2SO4 → CuSO4 + SO2↑ + 2H2O
Thus, we may observe copper wire oxidation by sulfuric acid formed from sodium hydrosulfate hydrate. The resulting copper sulfate, in the presence of water, provides a blue color to the reaction mixture.
Why does concentrated sulfuric acid oxidize copper?
It is well known that only concentrated sulfuric acid H2SO4 can withdraw electrons from metallic copper. If we dilute the acid, the reaction doesn’t proceed. In order to better understand this phenomenon, let’s consider surroundings of a sulfur atom in both cases.
When sulfuric acid is diluted, it is completely dissociated, i.e. represented by ions that to a certain degree freely move in a solution:
H2SO4 → 2H+ + SO42–
In this case, sulfur resides in a sulfate ion of a symmetrical geometry:

It should be noted that electrons (and with them a negative charge) are drawn to oxygen atoms. Sulfur atoms, in turn, carry a positive charge. Theoretically, sulfur atoms could withdraw electrons, too, but they are surrounded by oxygen atoms from all sides, and electrons simply cannot reach them. As a result, a sulfate ion is not a strong enough oxidizer to withdraw electrons from metallic copper.
On the contrary, in concentrated sulfuric acid, nondissociated neutral H2SO4 molecules prevail. Their geometry resembles sulfate ion structure, but it is somewhat distorted because two oxygen atoms have two hydrogen atoms connected to them:

Here, central sulfur atoms are not screened as sufficiently as they are in case of sulfate ions. As a result, concentrated sulfuric acid may withdraw electrons from metallic copper. Check it out:

That’s interesting!
How is copper sulfate obtained in industry?
Copper sulfate CuSO4 found its application in various fields of human activity. For instance, anhydrous copper sulfate can be used as a dehumidifying agent. Copper sulfate solutions may serve as an antiseptic and as a source for depositing copper on metals. It is also used in manufacturing high-purity metallic copper. Thus, copper sulfate is produced on an industrial scale.
One method for copper sulfate production is oxidation of metallic copper in diluted sulfuric acid saturated with air through a gas bubbler:
2Cu + O2 + 2H2SO4 → 2CuSO4 + 2H2O
Only rarely this reaction is used to purify naturally occurring copper. Normally, this method is applied for already remelted or alternatively refined copper, which is initially obtained by recycling non-ferrous scrap metal.
Another method for copper sulfate production is dissolving copper oxide in sulfuric acid. And copper oxide, in turn, is obtained via copper sulfide calcination:
2CuS + 3O2 → 2CuO + 2SO2↑
CuO + H2SO4 → CuSO4 + H2O
Moreover, sulfide may be immediately converted into sulfate by means of so-called firing sulphatization:
CuS + 2O2 → CuSO4
Starting materials – copper oxide and sulfide – occur naturally in form of ores. They can also be easily obtained by recycling copper scrap.
A choice of copper sulfate production method is often defined by location. If a factory is situated within city limits or nearby, then a starting material would be non-ferrous scrap containing a lot of copper. However, if a production is located near a mine, then copper ores serve as a raw material.