The characteristics of copper, and the reaction of the metal with nitric acid
Stable metal Vs. Strong oxidizer

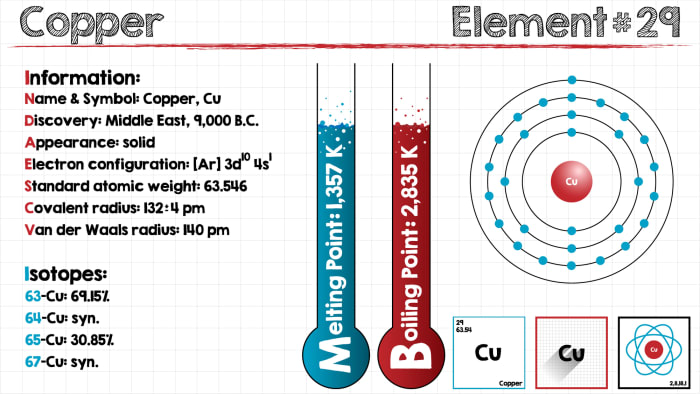
Copper is one of the oldest known metals, which has been used by people from ancient times. In Latin, copper is known as cuprum, and its atomic number is 29. In Mendeleev’s periodic table, copper is located in the fourth period, in the first group.
Physical and chemical properties of copper
Naturally occurring copper is a heavy metal of pink-red color with a ductile and soft structure. The boiling temperature is over 1,000 degrees Celsius. Cuprum is a good conductor of electricity and heat, and melts at a temperature of 1,084 degrees Celsius. The density of the metal is 8.9 g/cm3, and in nature it is encountered in its basic form. According to the electron formula of the copper atom, it has 4 levels. In the 4-s valence orbital there is one electron. In a chemical interaction with other substances, one to three negatively charged particles (electrons) split away from the atom, as a result of which copper compounds form with a degree of oxidation of +3, +2, +1. The maximum stability is displayed by divalent derivatives of copper.
Copper is a substance with a low capacity to interact. There are two main degrees of oxidation of the metal displayed in compounds: +1 and +2. Substances in which these values change to +3 are encountered rarely. Copper interacts with carbon dioxide, air, hydrochloric acid and other compounds at very high temperatures. A protective oxide film forms on the surface of the metal. This metal protects the copper from further oxidation, makes it stable and gives the metal a low activity.
Metal interacts with simple substances – halogens, selenium, sulfur. The metal is capable of forming double salts or complex compounds. Almost all the complex compounds of this element are poisonous, apart from oxides. Substances that are formed by monovalent copper easily oxidize to divalent equivalents.
In chemical reactions copper acts as a low-activity metal. The metal does not dissolve in water in ordinary conditions. In dry air the metal does not corrode, but when heated the surface of copper is covered with a black coating of oxide. The chemical stability of the element is shown in its resistance to impact of carbon, dry gases, several organic compounds, alcohols and phenol resins. For copper, complex reactions are characteristic, in which colored compounds are released. Copper has similarities with metals of the alkaline group, as it forms monovalent derivatives.
Copper — reaction with nitric acid
Copper dissolves in nitric acid. This reaction takes place because the metal oxidizes with a strong reagent.

Nitric acid (diluted and concentrated) displays oxidizing properties, with the dissolution of copper. In the reaction of the metal with diluted acid, copper nitrate and nitrogen divalent oxide form in the ratio of 75% and 25%. The equation of the reaction is
8HNO₃ + 3Cu → 3Cu(NO₃)₂ + 2NO + 4H₂O
In the reaction process, 1 mole of copper and 3 moles of concentrated nitric acid take part. When the copper is dissolved, the solution heats up intensely, the thermal breakdown of the oxidizer takes place, and additional nitric oxide is released. The equation of the reaction is
4HNO₃ + Cu → Cu(NO₃) + 2NO₂ + 2H₂O
This method of dissolving copper has its drawbacks – in the reaction of copper with nitric acid, a large amount of nitric oxide is released. To capture or neutralize nitric oxide, special equipment is required, so this process is too expensive. The dissolution of copper in nitric acid is considered complete when volatile nitric oxides stop being produced. The reaction temperature is from 60 to 70 degrees Celsius. The next stage is draining the solution from the chemical reactor. Pieces of copper remain at the bottom of the reactor, which did not enter into the reaction. Water is added to the liquid obtained, and it is filtered. Click here for learning properties of copper illustrated in interactions with other substances.
The reaction of nitric acid and copper illustrated by an experiment
The entire reaction of nitric acid and copper can be followed with the help of an experiment: place a piece of copper in concentrated nitric acid. A brown gas is released – first slowly, then more intensely. The solution turns green. If you add plenty of copper in the reaction process, the solution gradually turns blue. The reaction of copper with nitric acid takes place with the release of heat and toxic gas, which has an acrid odor. The reaction of copper and concentrated nitric acid is an oxidative-reductive reaction. The reducer sis the metal, and the oxidizer is nitric acid. The equation of the reaction is
Cu + 4HNO₃ = Cu(NO₃)₂ + 2NO₂↑ + 2H₂O
The reaction is exothermic, so in the spontaneous heating of the mixture it accelerates. The reaction of copper with nitric acid starts at room temperature. The metal is covered with bubbles, which start to rise to the surface and fill the test tube with brown gas – NO₂ (toxic poisonous nitrogen dioxide with an acrid odor). This gas is 1.5 times heavier than air. The reaction of copper with nitric acid takes place in two stages: at the first stage, the acid oxidizes the copper to copper oxide, releasing nitrogen dioxide; at the second stage, copper oxide reacts with new portions of acid, forming copper nitrate Cu(NO₃)₂. The mixture heats up, and the reaction accelerates.

The result is that the metal dissolves, and a solution of copper nitrate forms. The copper nitrate gives the solution a green or blue color (this will depend on the amount of water used).