Dyeing wool with tea
Dye a wool thread with tea and fixate color with iron(II) sulfate

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
You may use both green and black tea. Try different kinds to see which type of tea will color the fabric more effectively!
If you used black tea for the reaction, then the thread should have got colored. To make sure it did, take a new thread from the kit and compare its color with the one that seems uncolored.
After coloring with green tea the thread will have almost the same color as it had in the beginning.
In fact, iron sulfate intensifies the color. However, if we don’t add FeSO4 then the resulting color depends solely on tea or, to be more precise, on its type. The lighter the brewed tea, the weaker the resulting color of a thread.
Step-by-step instructions
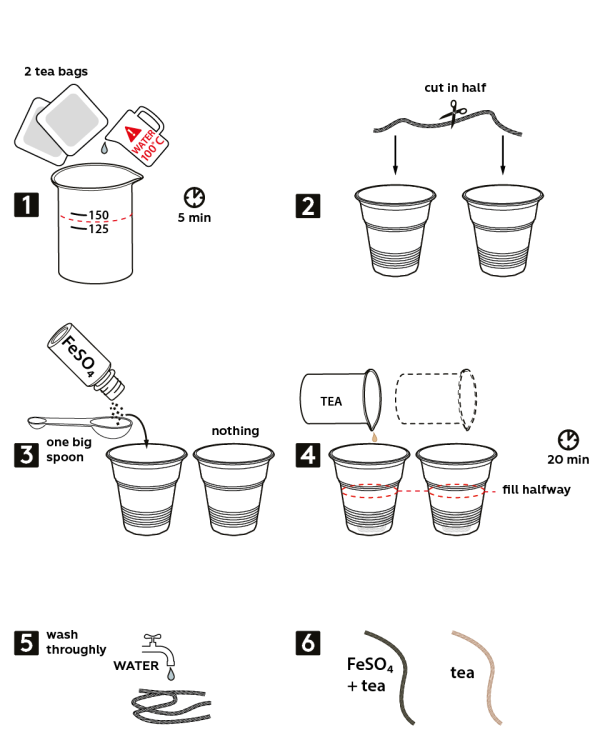
- Brew 2 tea bags in about 140 mL of boiling water. Wait 5 min.
- Take a woolen thread, cut it in half, and place the threads into 2 disposable cups.
- Pour one big measuring spoon of iron sulfate FeSO4 into the first cup.
- Fill both cups with tea (approximately half-way) and wait 20 min.
- Remove both threads from the cups and thoroughly rinse them with water.
- Compare the samples. The thread that had been treated with iron sulfate became black, and the other thread that has only been immersed in tea turned light-brown.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
What happens to the string and iron (II) sulfate?
The string is made of wool. Being a fabric of animal origin, wool contains proteins.
Proteins are very long and complicated molecules, twisted into several spirals, which contain many different segments. These segments are amino acids – organic compounds with carboxyl (–COOH) and amino (–NH2) groups. Amino acids are critical to any living creature. In fact, they are responsible for most of the biological processes in every living creature. For example, hemoglobin, which transfers oxygen to all parts of the human body, is a protein.
Iron ions are very attracted to tannins – one of the main chemicals which give tea its dark color. When we pour tea in the cup with iron sulfate, it immediately darkens because of the complex compound iron ions and tannins form. At the same time, due to the presence of iron ions, such compounds react with the proteins in the wool.
Therefore, when a string of wool is put in a solution of tea and iron(II)sulfate FeSO4, the compound made of iron ions and tannins binds to the wool. This gives the wool a water resistant dark brown color.
In this experiment, iron (II) sulfate is a mordant. Mordants are chemical compounds which strongly connect or join molecules of dyes and wool. In other words, mordants act as a special kind of linking molecule.
The most common substances of this sort are alums. A typical alum molecule always has an ion with single positive charge, such as potassium K+ or ammonium NH4+, an ion with triple positive charge, for example, chromium Cr3+ or aluminum Al3+, and sulfate anion SO42–. Alums can be found in nature and are believed to be among the safest mordants. An example of a natural mordant is potassium alum KAl(SO4)2∙12H2O.
Why do the two strings differ so much?
The string, which was in the cup with both the tea and iron(II) sulfate, has an intense and water resistant color. This is due to the iron(II) sulfate which acts as a mordant. Iron(II) strongly holds the tannins and does not let them wash out of the wool.
Using this explanation, the string, which was in the cup with only the tea and no additives, has no substances which could bind the tannins to wool properly. This is why it loses most of its color after washing.