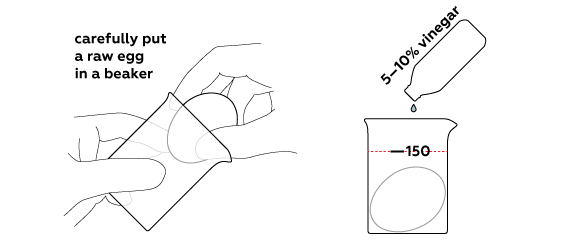
Rubber egg
Dissolve an eggshell using vinegar


Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
For this experiment, it is best to use regular chicken eggs with white eggshell. A brownish or variegated eggshell dissolves noticeably worse.
Quail eggs would also work very well for the “Rubber egg” experiment.
Normally, vinegar is sold as an essence (70% concentration) or as a 30% solution. However, the higher the concentration of the solution, the slower the reaction would go. Thus, you should prepare your own 10% vinegar.
If you’re working with a 70% solution, take 20 mL of it and dilute with 140 mL of water. Use a flask to measure volume.
And if you’re working with a 30% solution, in that case, pour 40 mL of it into a beaker and dilute with 120 mL of water.
Step-by-step instructions
Egg shell consists mostly of calcium carbonate CaCO₃, which readily reacts with many acids. We’ll use acetic acid, which is the main component of vinegar.
The reaction between acid and calcium carbonate produces carbon dioxide CO₂ gas. Its bubbles force the egg out of the solution. Let’s push it back down to completely immerse the egg in acid and wait some time.
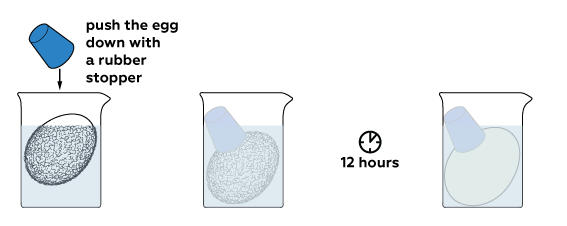
Calcium acetate is obtained in this reaction; it is highly soluble in water. That’s why almost nothing remains from the egg shell. Rinse off what is left with water. Here is your “rubber” egg!
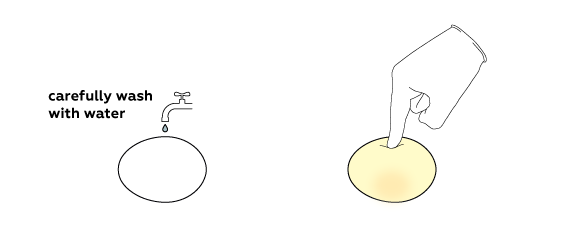
If the shell is very thick or the egg is very large, the amount of acid used might be insufficient to completely remove the shell. In this case immerse the egg in fresh vinegar and wait a bit more.
Expected result
Acetic acid CH3COOH reacts with calcium carbonate CaCO3 contained in an egg shell, thus dissolving the latter. This reaction yields calcium acetate. The egg itself becomes soft and rubbery to touch.
Disposal
Pour solutions down the sink. Wash away with an excess of water.
Scientific description
What happened to the egg shell?
Insoluble calcium carbonate (CaCO3) that makes up the outer layer of the shell eggs reacted with vinegar - an aqueous acetic acid solution (CH3COOH). As a result, soluble calcium acetate (Ca(CH3 COO)2) was formed. The gas bubbles evolved in the process is carbon dioxide (CO2), excluded from the carbonate by the acid. So, what happened with the egg shell can be expressed by the reaction equation:
CaCO3 + 2CH3COOH → Ca(CH3COO)2 + CO2↑ + H2O
The result is an egg without a hard shell. Only the inner shell membranes, which are similar to parchment (the same matte film, which can be seen when cleaning boiled eggs) remain intact. They are strong enough to hold the liquid contents inside, but are still quite fragile. Therefore, the egg should be handled most carefully. If it is dropped or inadvertently squeeze, it would be necessary to do unscheduled cleaning!
Calcium carbonate is part of the shell in three forms. Straight above the inner shell membranes is calcium carbonate in the form of calcite. It has a pronounced crystalline structure and does not dissolve in water. Above it there is a layer of aragonite - a different crystalline modification CaCO3. It has slightly higher solubility. On the surface is amorphous vaterite - the soluble form of calcium carbonate. When the mechanism of shell formation fails, a bird can only lay eggs with vaterite in them. Such eggs are fragile and poorly protect the chick from external factors. If the shell consists of calcite, gas exchange in it иbecomes difficult, so does the evaporation of excess moisture. In addition, it is extremely difficult for the chick to crack the egg from inside and hatch.
Why does the egg increase in size?
If you take the egg which was "peeled" by acid and compare it with a fresh one, it will be clearly seen that the size of the egg treated with vinegar is much more. You can even measure and write down the length and width of the product before and after the experiment, and then calculate the difference. This is due to the fact that the treated egg contains more fluid. Elastic inner shell membranes remaining on the surface after treatment with vinegar let water through, which "goes after” sodium ions Na+. Also the water "attracts" protein. As a result, the egg increases in volume. The difference is even more noticeable in case of the addition of some colorants to the vinegar. In the following video they obtained a glowing egg, using a highlighter ink as a colorant.
The increase in the volume of eggs is due to the specific structure of inner shell membranes. In a normal egg their special structure allows the developing chick to breathe and move excess moisture that forms during the breaking down of fats and proteins outside the shell. In fact, these membranes are semipermeable. They let some substances through, while remain impermeable to others. Most easily the shell lets through water.
During the experiment, the water flows inside, trying to "dissolve" the protein. Furthermore, the concentration of sodium ions tends to come to level on both sides of the membrane, at that ions themselves cannot pass through the shell. Therefore, the water of vinegar (which contain no sodium ions) gets into the egg (inside the concentration of sodium ion in NaCl equivalents is 0.9%).
If prior to the experiment you slightly salt the vinegar with regular table salt, the size of the egg will not change after the shell dissolves. If the amount of salt added to the acid is greater, in the course of the experiment the egg even wrinkles, since the water from it will flow to the area of higher concentration of sodium ions. By the way, when cooking eggs the water is slightly salted exactly in order to avoid a rapid expansion of the content. If you cook them in fresh water, the likelihood that the shell will crack in the process will be greater.
What eggs are best to use in the experiment?
It is best to use a common raw egg with a white shell for the experiment. There is sufficiently less organic compounds that inhibit the interaction with vinegar on the surface of such eggs. The dissolution of an egg shell with dark coloring will be slightly slower. If you do not want to wait long for the result, you can use a quail egg. Its thin shell is "dissolved" in vinegar very quickly. Any poultry egg is suitable for this experiment. Do not use the eggs of wild birds, though, because that's inhumane.
But not only humanity is the reason of the recommendation to avoid the use of eggs of wild birds. Some of them are totally unsuitable for the experiment.
It would be very difficult to carry out the experiment with eggs of birds such as flamingos (Phoenicopterus ruber), grebes (Podicipedidae) and malleefowls (Leipoa ocellata). The reason is that, instead of calcium carbonate, their shells are made up of calcium phosphate (Ca3(PO4)2). Calcium phosphate reacts very slowly with acetic acid:
Ca3(PO4)2 + 4CH3COOH ↔ Ca(H2PO4)2 + 2Ca(CH3COO)2
Due to the fact that the reaction is reversible, and the products remain in the reaction mixture (in contrast to the reaction of calcium carbonate with vinegar, where the carbon dioxide leaves the reaction zone), the process of dissolving a phosphate shell takes very much time.
There are also poultry egg shells that consist of calcium carbonate, but the top layer is covered with calcium phosphate. These are feathered cormorants, northern gannets (Morus bassanus), australian bushturkeys (Alectura lathami), emperor penguins, frigates. Their eggs we also do not recommend for the experiment.
Eggs that are covered with a phosphate shell are layed by birds, forced to nest in conditions of constant humidity. Due to its poor solubility and low wettability, such a shell protects the developing chick in adverse conditions best.
Follow up
Quail eggs
Try using quail eggs for this experiment. Thanks to their size, color, and some other features in composition and structure, their shells will dissolve much faster. Their appearance will also differ. Compare results!
An eggshell “breathes”!
This experiment will assure you that an eggshell is really not a hard impermeable cage for a future chick. Indeed, it has numerous pores. In fact, an eggshell is a tiny and durable filter! Interestingly, these pores are unevenly distributed over an eggshell surface, and you'll see it for yourself.
To do so, take a regular medium-sized chicken egg, two shot glasses (or other glassware suitable for holding eggshell halves), a beaker, a tablespoon, a big syringe from the Starter kit (or a teaspoon), red fountain pen ink or India ink, and water. You may also use eosin (food color) instead of ink. You may have already used it in one of our experiment sets. Take into account that pastel, watercolor, and acrylic paint are not suitable for this experiment. Also, other food colors or different color inks might not work here, too.
Carefully crack an egg into halves (as if you were cooking an omelet) and pour its contents out—we won’t need it for this experiment (why not cook an omelet, indeed?). What we need is two eggshell halves: one is pointy, and the other is rounded. Carefully but thoroughly wash both halves to clean off any residues and then place them in shot glasses, cracked sides up. Now, they remind two bowls made of eggshells. Make sure there are no cracks or holes in them: otherwise, our experiment will be pointless.
Fill the beaker quarter-full with warm water; add a tablespoon of ink and stir. If using eosin, add enough water to get a vibrant red color. Then, pour even amounts of the colored liquid into both eggshell halves (use a syringe or a teaspoon for measuring—choose whatever method is more convenient for you). And now measure time! Soon enough the red liquid will penetrate through pores in the eggshells and leak into the shot glasses. Interestingly, the rounded eggshell half will be emptied faster than the other half. In fact, there are almost twice as many pores on the rounded end than on the pointed end of an egg.
That’s interesting!
What is an eggshell like?
The shell of an egg is a very complex structure. It does not just prevent the liquid contents from spilling out, but also performs many important functions.
First, the shell provides the contents physical protection from mechanical damage. And it's not just because the shell is hard. Its form is also of paramount importance. For example, the eggs of birds that nest on cliffs have a stiffening rib- a longitudinal thickening of the shell. This is to ensure the bird does not crush its future offspring with its weight when it lands on the clutch. Besides, such eggs often have a strongly pointed end which prevents the egg from rolling away too far.
Second, the inner part of the shell serves as a source of calcium for forming the skeleton of the chick. The composition of bone tissue includes a large amount of calcium phosphate (Ca3(PO_ {4})2), so a healthy chick will not be able to develop inside an egg with no shell.
Third, the shell is covered with a great number of pores. Yes indeed, it is not a solid impermeable structure! Air and water can pass through these pores. This can easily be seen. To check how air passes through the natural ostioles of the shell, you need to lower the egg into hot water. A lot of bubbles will quickly appear on the surface. This happens because there is some air inside the egg. And the fresher the egg, the smaller the air pocket in it. When heated gases greatly expand and seep through the pores. To determine the permeability of the shell to liquid, as well as that the pores are distributed unevenly, one has to conduct a bit more difficult experiment, which is described in detail in the "follow up" section. There are almost twice more microscopic holes at the blunt end of the egg than at the point end. The total number of pores in the shell of an egg amount to 13,000.
Can the egg be eaten after the experience?
One better withhold from eating such an egg. With time vinegar gradually passes through the shell, what one can make sure of by simply smelling the contents. But at the same time this vinegar does not pose any real danger. If you are an experienced cook, you can use the yolk for making "Provencal" sauce (mayonnaise). But still you should say goodbye to the egg white.