Atom structure
In this lesson, you will learn that an atom consists of a tiny atomic nucleus surrounded by an electron cloud. We will discuss the three main subatomic particles: protons, neutrons and electrons, and their properties.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

In the previous lessons, we have seen that all matter is built out of small atoms. But what are atoms themselves? What's inside them?
We will start with our helium balloon.
Let's look inside. Ready to dive?

We have to zoom in a billion times to see the individual helium atoms.
Let's choose one of those atoms and get closer.

You see that this atom consists of an atomic nucleus surrounded by an electron cloud. That is how atoms are made.

The nucleus is positively charged and electrons are negatively charged, so they attract each other.

The real nucleus size is much smaller than you see here. Let's show its real size.
It is now smaller than one pixel on your screen.
It is about hundred thousand times smaller than the size of an atom.

Nevertheless, almost all of the atom's mass, over 99.9% of it, is found in the nucleus.

Let's zoom in to get a good look at this nucleus.

Now you can see that the nucleus consists of protons and neutrons tightly bound to each other.
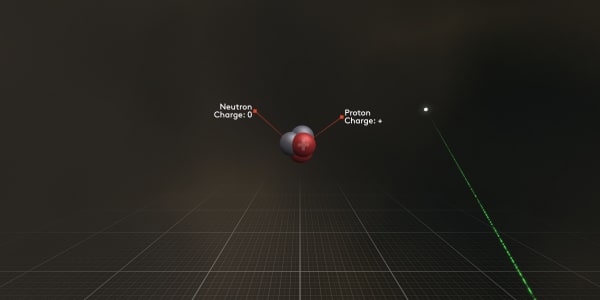
The protons are positively charged so they actually attract electrons.
The neutrons are not charged at all.

Let's go back to our atom.
Later, we will show the nucleus blown up, just to make it easier to see. Just remember that an actual nucleus is much much smaller.

Now we will look at another atom.
Please point at the atomic nucleus.
Now point at the electrons.

Now, let's get closer to the atomic nucleus again.
Point at a proton.
Protons are positively charged; you can see a small plus sign on them.
Now point at a neutron.
A neutron has zero charge. Protons and neutrons are almost of equal mass and both are more than a thousand times heavier than an electron.

Let's go back to our laboratory.
Which of these particles attracts a proton?

A negatively charged electron will attract a positively charged proton.

Which of these particles will be repelled by a proton?

Same charged particles will repel one another. So, two positively charged protons will repel each other.
Teacher's notes
Keywords
atoms, electrons, nucleus, protons, neutrons, coulomb forces
Common misconceptions
- Gravity is one of the forces pulling nuclei and electrons together.
- Planetary model: electrons move around the nucleus.
Students will
- Learn that the atom consists of a positively charged nucleus and negatively charged electrons
- Learn that a nucleus consists of protons and neutrons
- Learn about the forces that bring nucleus and electrons together
- See that electrons spread around the nucleus like a cloud
- Learn that the nucleus is much heavier than electrons
- Learn the relative distance from the nucleus to electrons compared to nucleus size
Hands-on activities
After VR
The aim is to give students an idea that the nucleus is much heavier than electrons.
Ask students to weigh a 2-litre bottle of water. Then calculate the weight of an electron, assuming that the bottle weight represents the mass of a proton. Ask students to weigh a piece of playdough representing an electron.
History and sources of knowledge
- Old theories that atoms are not divisible.
- Thomson’s discovery of electrons.
- Rutherford gold-foil experiment.
Topics to discuss
- How and where atoms are formed.
- It’s not correct to say that atoms are mostly an empty space.
- The weight of protons and neutrons as compared to electrons.
Fun facts and quotes
- The largest animal that now exists on Earth is a blue whale. Its length is up to 30 metres and a weight of up to 180 tons. If we replace all the atoms that form a blue whale with their nucleus the length of the animal would be 0.3 mm (it's close to the smallest object we can see with naked eyes). And the weight would not change at all.
- In neutron stars, all matter is denser (up to 10 times) than in an atom nucleus.
- The force of gravity between the nucleus and electrons is 1 and 42 zeros weaker than the electrostatic force actually holding them together.
- Most of the atoms in the universe are from supernova explosions.
Questions
- Name subatomic particles.
- Which subatomic particles are charged?
- Which subatomic particles are not charged?
- Which is heavier electron or neutron?
- Which is heavier electron or proton?
- What is the force holding nucleus and electrons together?
Calculating
The electron is approximately 2,000 times lighter than a proton. Calculate the mass of an electron as a percentage of the proton mass.