Atoms in solids
The first lesson shows that all matter consists of atoms. It will take students inside graphite and a diamond. Students will see that both materials consist of the same carbon atoms, but have very different properties because their atomic level structure is different. Students will also learn that atoms in solids do not stay still – they vibrate.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript
Welcome to the MEL Virtual Laboratory, where you can see the invisible.
Today you will use our virtual microscope for the first time.
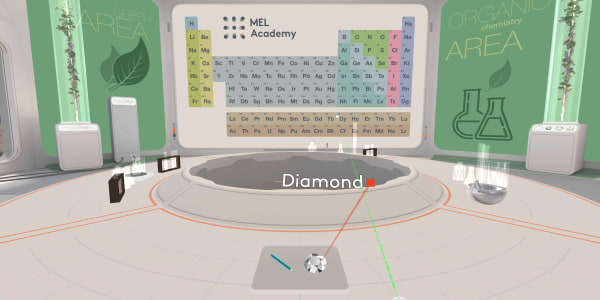
We will start with a diamond.
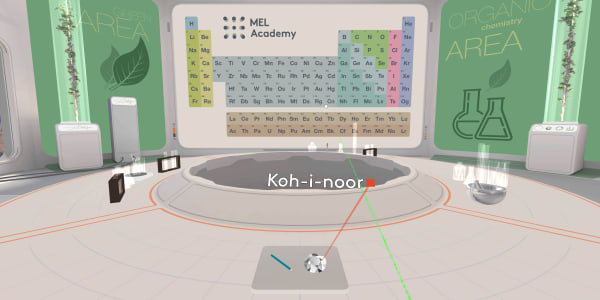
This diamond is called the Koh-i-Noor.
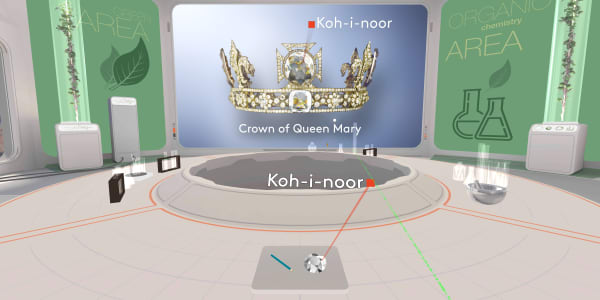
It was found in India about a thousand years ago. People estimate its value to be over a billion dollars.
Let's look inside. Ready to dive?
We will zoom in. Each cell you see is ten times smaller than the one before. We need to zoom in about a billion times. That’s nine zeros after a one!
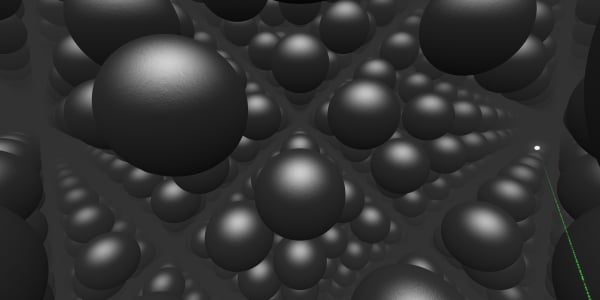
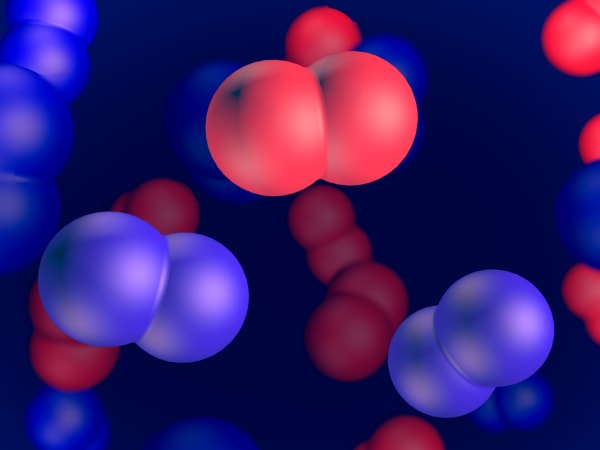
And now we see these small particles that our diamond is built of. They're called atoms.
But in real life they do not stay still. They are vibrating.
Let's switch time on. Ready, steady, go…
All matter is built of atoms that are constantly moving. In solids, like our diamonds, they vibrate.
You can see how each atom is connected to four others. Such a strong structure makes a diamond one of the hardest materials.
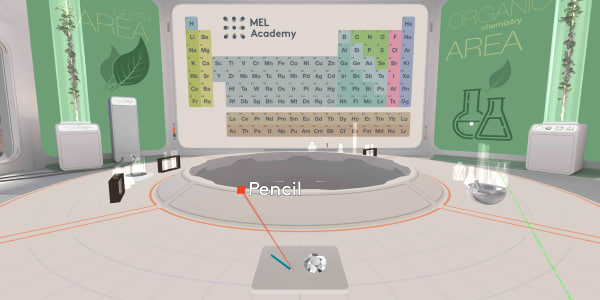
Let's return back to our laboratory.
We also have a pencil here. It's a billion times cheaper, but interestingly its lead is made of the same carbon atoms as a diamond.
Let's look inside. Ready to dive?
We have to zoom in about a billion times to see the individual atoms.
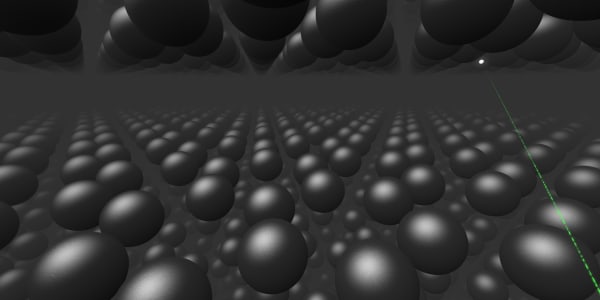
They are the same carbon atoms as in a diamond but their arrangement is different. As you can see, they are laid out in layers. These layers are easy to separate from each other, since there is no strong bond between them.
This makes graphite much softer than a diamond. When you write with your pencil, the marks on the paper are traces of the graphite.
You can fly inside this graphite crystal and explore it by yourself.
Just to remind you, you are now as small as an atom and what you see around you is the tip of a pencil enlarged a billion times.
Let's go back to our laboratory.
You have seen that both diamond and graphite are built of the same atoms but they have very different properties because of their atomic structure.
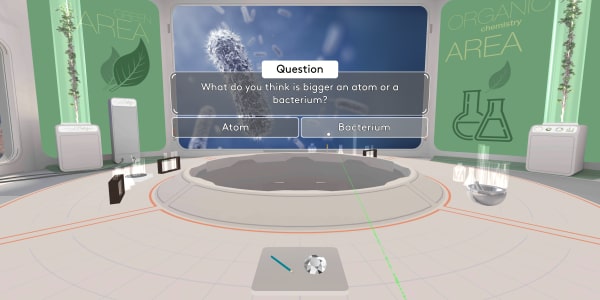
Try to recall how small the atoms are. What do you think is bigger, an atom or a bacterium?

A bacterium is much, much bigger than an atom. Each bacterium contains billions of atoms.
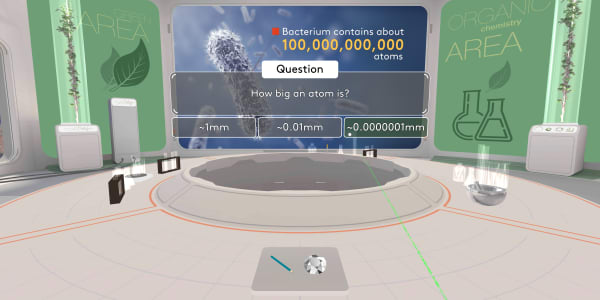
Now, try to think how big an atom is?

An atom is about ten millions times smaller than a millimeter.
If an atom were the size of an ant, then this ant would be the size of Manhattan.
Teacher's notes
Keywords
atoms, matter, state of matter, solids, matter properties
Common misconceptions
- Atoms in solids don't move.
Students will
- Learn that matter consists of atoms
- Learn that atoms in solids are packed close together and remain in the same place
- Find out that atoms in solids are constantly vibrating
- See that different arrangements of the same type of atoms lead to different properties in solids
- Compare the size of atoms to other objects
Hands-on activities
After VR
The aim is to show the students that the structure of graphite (seen in VR) is what allows pencils to write. As we write, layers of graphite are mechanically peeled off the surface of the pencil core. As graphite is a conductor, we can see that a pencil line can connect an electrical circuit.
Ask students to connect an electrical circuit with a pencil line. The diode will light up. Let students try to explain why this happens.
History and sources
of knowledge
- From ancient Greeks to Dalton's theory and modern days.
- Modern techniques to see atoms: scanning tunneling microscope – image of a single-wall carbon nanotube:
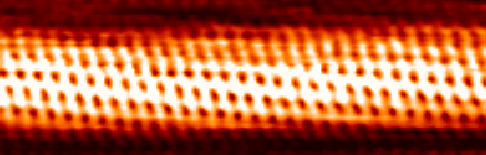 .
.
Topics to discuss
- How can we trust theories about things we can't see?
- The best scientific explanation is based on evidence (observations) and scientific knowledge.
Fun facts and quotes
- How small atoms are: If atoms were the size of an apple, an apple would be bigger than the Earth.
- A copper penny contains a trillion times (1,000,000,000,000) more atoms than there are people on the Earth (which is greater than 7 billion).
From the lectures of Nobel Prize winner Richard Feynman:
“If, in some cataclysm, all of the scientific knowledge were to be destroyed and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.”
Questions
- Name something around you that consists of atoms.
- Name something that doesn't consist of atoms.
Calculating
Count how long a line of 1,000,000 sulfur atoms would be if arranged side by side, if a single sulfur atom is 200 pm (0.0000000002 m).
Quiz
Please see below for the link to a Google form containing a quiz on the material above.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.