Characteristics of potassium and its interaction with water
Why it cannot be kept in the open air

Potassium is the 19th element on the periodic table, and is an alkaline metal. It is a simple substance which in normal conditions is in a solid aggregate state. Potassium boils at a temperature of 761 degrees Celsius. The melting temperature of the element is 63 degrees Celsius. Potassium has a silvery-white color, and a metallic shine.
The chemical properties of potassium
Potassium is a chemical element with a high chemical activity, so it cannot be kept in the open air – the alkaline metal immediately enters into a reaction with surrounding substances. This chemical element belongs to the 1st group and 4th period of the periodic table. Potassium has all of the properties characteristics for metals.
Potassium interacts with simple substances, such as halogens (bromine, chlorine, fluorine, iodine), and also phosphorous, sulfur, nitrogen and oxygen. The interaction of calcium with oxygen is called oxidation. In this chemical reaction, oxygen and potassium are consumed in the molecular ratio of 4 parts to 1, as a result of which two moles of potassium oxide form. This interaction can be expressed by the equation:
4К + О₂ = 2К₂О
When potassium burns, it has a bright purple flame. This reaction is considered qualitative for the determination of potassium. The reaction of potassium with halogens is named in accordance with the names of the chemical elements: fluorination, iodination, bromination and chlorination. These interactions are called combination reactions, as the atoms of two different substances unite into one. For example, the reaction between potassium and chlorine, which forms potassium chloride. To carry out this reaction, take two moles of potassium and one mole of chlorine. As a result, two moles of the potassium compound form:
2К + СІ₂ = 2КСІ

With nitrogen, potassium forms a compound when it burns in the open air. Potassium and nitrogen are consumed in this reaction in the molecular ratio of 6 parts to 1, as a result of which two moles of potassium nitride form:
6К + N₂ = 2K₃N
The compound consists of crystals of a greenish-black color. Potassium reacts with phosphorous according to the same principle. If you take 3 moles of potassium and 1 mole of phosphorous, you get 1 mole of phosphide:
3К + Р = К₃Р
Potassium reacts with hydrogen, forming a hydride
2К + Н₂ = 2КН
All the combination reactions take place at high temperatures.
Interaction of potassium with complex substances
Complex substances with which potassium enters into a reaction include water, salts, acids and oxides. As potassium is an active metal, it forces the hydrogen atoms out of their bonds. For example, the reaction between potassium and hydrochloric acid. Take 2 moles of potassium and 2 moles of acid. As a result of the reaction 2 moles of potassium chloride and 1 mole of hydrogen form:
2К + 2НСІ = 2КСІ + Н₂
We should look in more detail at the interaction of potassium with water. Potassium is an active metal which reacts violently with water. The element moves over the surface of the water, and is pushed by the hydrogen released:
2K + 2H₂O = 2KOH + H₂↑

During the course of the reaction, in one unit of time a great deal of heat is released, which leads to the ignition of the potassium and the released hydrogen. It is interesting to observe this process – the potassium instantaneously ignites on contact with water, a purple flame crackles and quickly moves on the surface of the water. At the end of the reaction there is a flash, spattering drips of burning potassium and reaction products. The main end product of the reaction of potassium with water is potassium hydroxide (an alkali). The formula is
4K + 2H₂O + O₂ = 4KOH
Warning! Don’t try to repeat this experiment without a professional supervision!
If the experiment showing the interaction of potassium with water is conducted unsuccessfully, then you may get burned by the alkaline. To conduct the reaction, we usually use a crystallizer with water, in which we place a piece of alkaline metal (potassium in this case). During this effective experiment, many observers try to get close to the laboratory table, and as soon as the hydrogen stops burning, they look into the crystallizer. At this moment, the final stage of the reaction of potassium with water takes place, accompanied by a small explosion and the splattering of the hot alkali that has formed. So for safety reasons, you should remain at a certain distance from the laboratory table, until the reaction has finished completely. Here you’ll find the safest experiments to do at home with your kids.
The structure of potassium
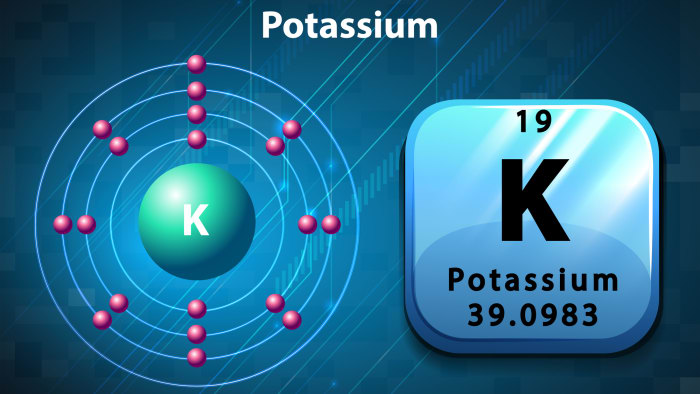
The potassium atom consists of a nucleus containing protons and neutrons, and electrons which revolve around it. The number of electrons is always equal to the number of protons in the nucleus. When an electron breaks away or when one joins an atom, the atom ceases to be neutral and turns into an ion. Ions are divided into cations and anions. Cations have a positive charge, and anions a negative charge. When an electron joins an atom, the atom turns into an anion, while if one of the electrons leaves its orbit, a neutral atom turns into a cation.
The atomic number of potassium in the periodic table is 19, and so there are also 19 protons in the nucleus of the element. Conclusion: there are 19 electrons revolving around the nucleus. The number of protons contained in the structure of the atom is determined as follows: subtract the atomic number of the element from the atomic mass. Conclusion: there are 20 protons in the nucleus of potassium. Potassium belongs to the 4th period, and has 4 “orbits”, and electrons are arranged evenly on the orbits, which are in constant movement. The diagram of potassium looks as follows: in the first “orbit” there are 2 electrons; there are eight in both the second and third, and on the fourth and last “orbit”, there is one electron. This explains the high level of chemical activity of potassium – its last “orbit” is not completely filled, so the element strives to bond with other atoms, as a result of which the electrons of the last orbits of two elements become shared.