Egyptian night
A solution is suddenly plunged into darkness!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
You can use about 10–20 mL of pasta water – it’s quite starchy! Raw potatoes are also high in starch. To extract the starch, grate half a cup of potatoes, pour boiling water over the grated mass, and wait for 10–15 min. And voila – you have starchy water suitable for the experiment!
The reaction rate is influenced by two main factors: the temperature and the concentration of the reagents. In this case, the water was likely too warm. To keep the solution from darkening immediately, use colder water or add more sodium thiosulfate.
Most likely, the water in the beaker was too cold. Repeat the experiment using room temperature water. Alternatively, to speed up the reaction, you can add less sodium thiosulfate – try using just two to three drops. Try different options and see which one is the most successful!
You may have added too much sodium thiosulfate solution. Another possible reason is a lack of starch. Repeat the experiment, carefully measuring the quantities of reagents and giving the starch more time to soak in the hot water.
This can happen if the starch didn’t dissolve very well. It could be that the water you used to dissolve it wasn’t hot enough. Try repeating the experiment, soaking the starch in water that has just recently boiled.
Step-by-step instructions
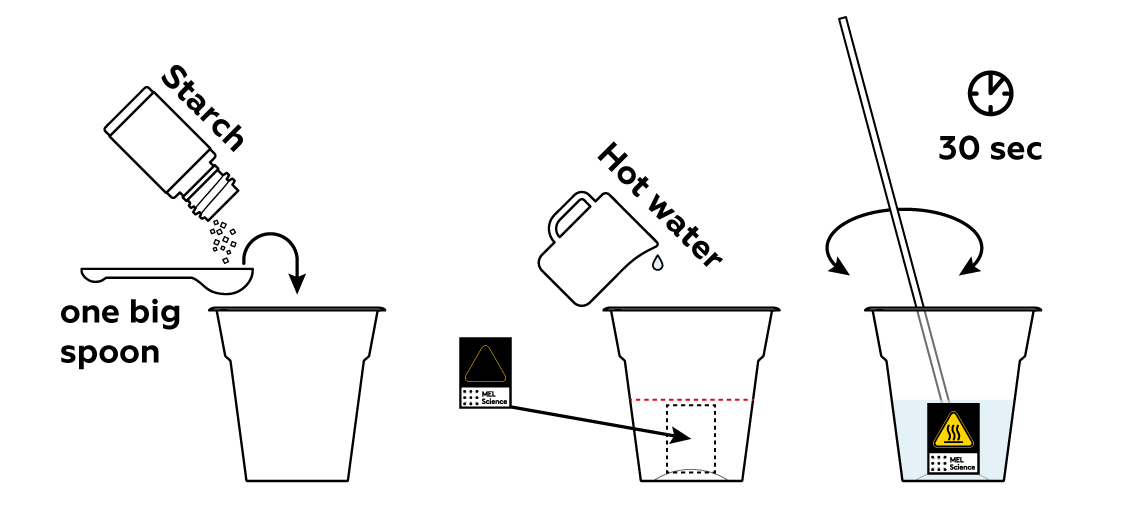
First, you need to prepare a starch solution. Starch is great at detecting iodine I2: it will turn dark blue if I2 is present. But as long as there's no I2, it will just sit there.
Also add KI, which yields iodide particles I-. I- is not I2, and starch won't detect it. And lastly, add Na2S2O3, which yields S2O32-. The Na2S2O3, KI, and starch solutions won't do anything notable just yet.
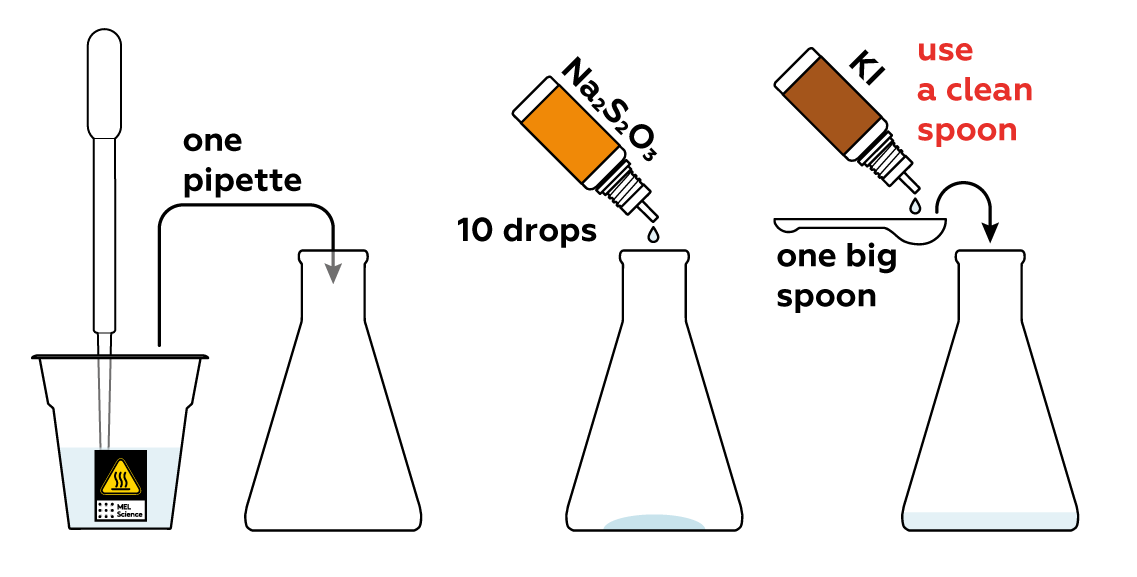
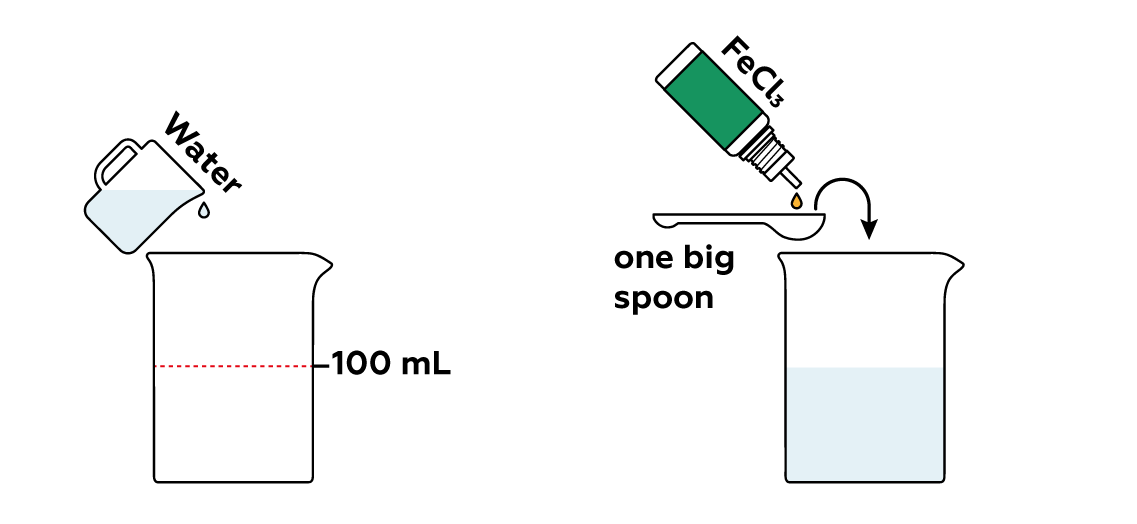
Meanwhile, pour some FeCl3 into the beaker. The Fe3+ from FeCl3 is really eager to grab electrons from I-, helping them combine to form iodine molecules I2.
Mix the liquids and don't blink!
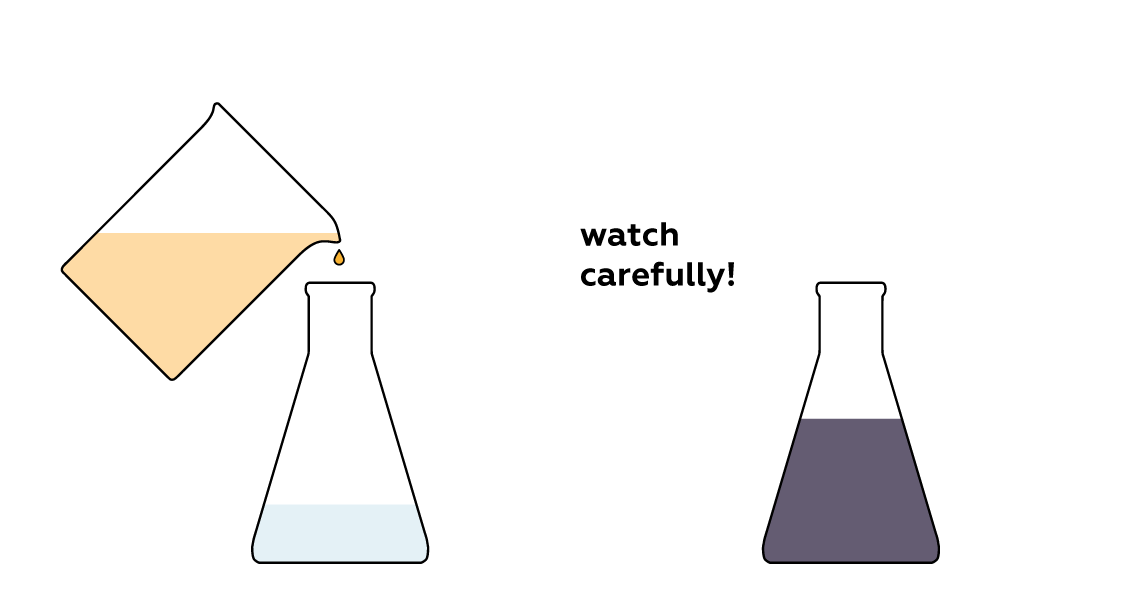
Expected result
The colorless solution in the beaker suddenly turns dark.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
Initially, not much seems to be going on, but a whole host of processes are churning away under the hood. Fe3+ 



is quickly turning them back into I−


are fighting for iodine's attention, but S2O32-
takes the lead.
But this state of affairs can’t last forever—both Fe3+ 
are slowly being used up in the process. However, there's much more Fe3+

. Once the S2O32-
is used up, Fe3+






How to change the reaction time?
This reaction is a version of chemical clock reactions – the moment when the solution changes its color can be calculated down to the second and depends on the quantity of reagents used for the reaction. The proportions of the substances used influence the rate of these changes. For instance, should we add half as much sodium thiosulfate, the solution will turn blue twice as slowly.
This experiment perfectly demonstrates the chemical law of mass action, in which the reaction 
. The process will continue until we run out of components.
That’s interesting!
Why is this experiment called Egyptian Night?
In Egypt, night falls rapidly and completely, leaving the sky very dark after a sudden sunset. In our experiment, we can observe as the solution turns dark blue in an instant. This is the source of the experiment’s poetic name.



