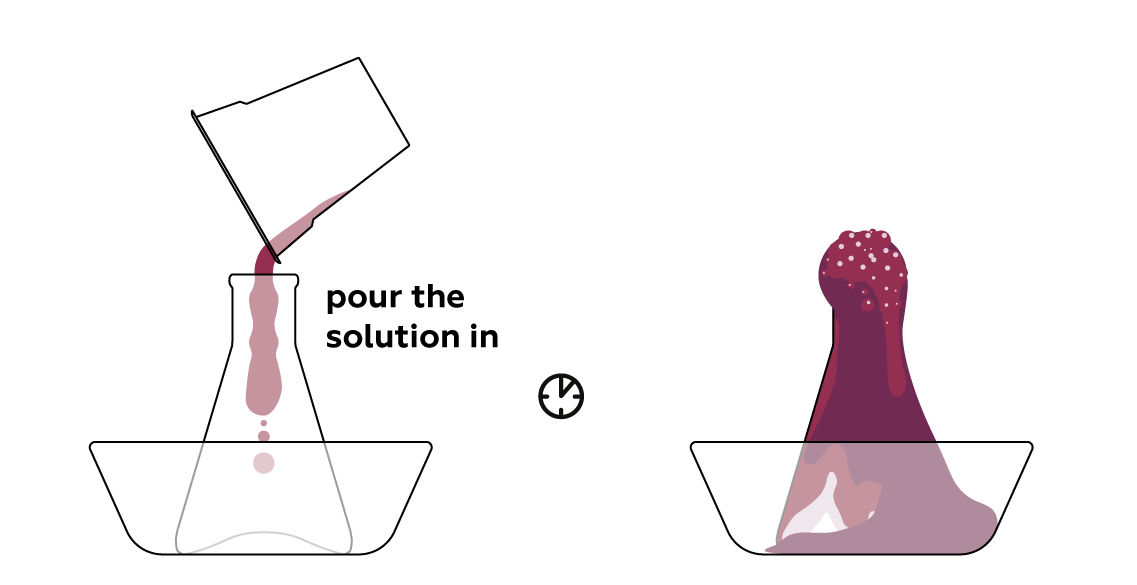
Foam eruption
Foam erupts out of the flask like real lava!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Yes, of course! You can continue the experiment with this plastic cup.
Pour some water into another empty cup. Make sure the syringe’s blue plunger is pressed all the way down. Place the tip of the syringe vertically in the water. Gradually pull the blue plunger till the black stopper is near the 10 mL mark. If any bubbles get trapped inside the syringe, you can either take a bit more water to compensate or return the water to the cup and try again.
Small particles of the reagent may stick to the walls of the cup. As long as almost all the anthocyanin has dissolved and colored the solution, this won’t make your result any less beautiful – feel free to continue the experiment!
Try shaking the flask horizontally. Don't shake it vertically or too intensively, or you might spill the contents!
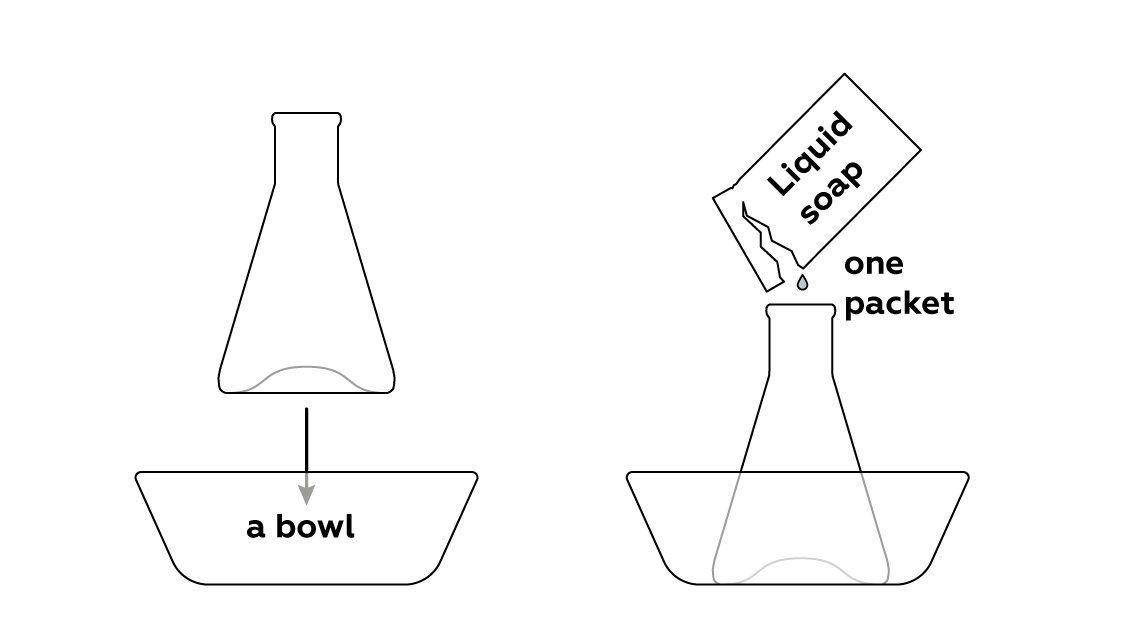
Your kit contains two packets of liquid soap, so you should be able to perform this experiment twice. You can use your own liquid soap, but we can’t guarantee the result. It probably won’t be as impressive, but it depends on the kind of liquid soap you use.
This experiment should create a lot of foam, so we recommend using a deep bowl. It’s great if the bowl is transparent – that way, it’s easier to fully evaluate your results!
All the compounds are absolutely safe, so you can wash them down your kitchen sink. Rinse the sink with water to remove every trace of the anthocyanin!
Step-by-step instructions
Prepare a solution of anthocyanin, a natural dye.
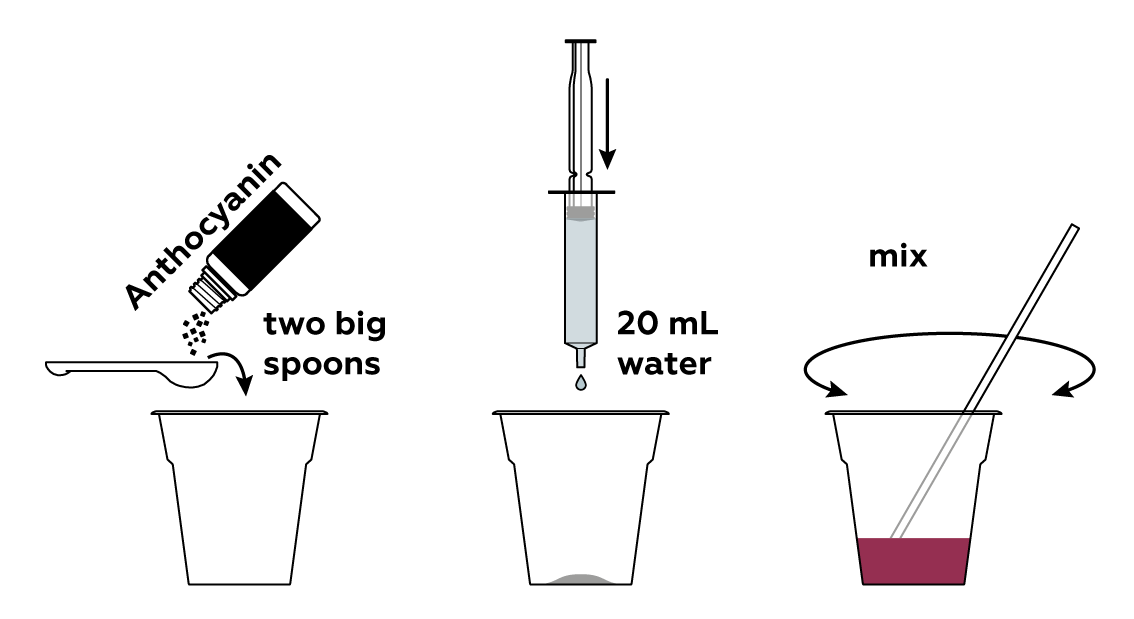
Prepare a dry reagent mixture, which will act as a source of carbon dioxide.

Add some liquid soap, which will later cause the mixture to foam.
Pour the dye solution into the dry reagent mixture to initiate the process.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description








, which easily decomposes into water H2O
and gaseous carbon dioxide CO2
. The latter fills the bubbles and makes the soap foam in the experiment.

What does this foam consist of?
The foam is simply a lot of carbon dioxide gas CO2 that makes the liquid soap form bubbles. Plus, of course, the food coloring anthocyanin, which can be found in grapeskin, and which makes the foam much more spectacular. You might also notice some particles of a white solid in the foam – these are small amounts of the initial powders (citric acid and sodium carbonate) which have neither reacted with one another nor dissolved in the water.
But how does this foam form?
As we’ve already figured out, the foam forms when a large amount of carbon dioxide gas bubbles vigorously through the liquid soap. But how does the carbon dioxide form?
Initially, we mix two substances: citric acid H3C6H5O7 and sodium carbonate Na2CO3. As solids, they don’t interact with each other. They react when we add water, or in this case, the water-based anthocyanin solution.
Both citric acid and sodium carbonate split into ions in water. Citric acid yields citrate anions C6H5O73– and hydrogen ions H+, while sodium carbonate yields carbonate anions CO32– and sodium cations Na+.
Each carbonate anion CO32– reacts with two hydrogen ions H+ to form carbonic acid H2CO3. Carbonic acid is unstable and easily decomposes, yielding water H2O and gaseous carbon dioxide CO2↑. These processes happen rather rapidly and a lot of gas forms in a very short time, causing the foam to erupt!
What are anthocyanins and what do we use them for?
Anthocyanins are natural dyes that are often used as food coloring. They are usually extracted from grapeskin, but can also be found in many other fruits, berries, and plants. Raspberries, blueberries, blackberries, and cranberries all owe their colors to anthocyanins!
We add anthocyanins to our experiment just for fun. Everything would work just as well without them, but our bubbles would be totally colorless without the first step (adding and dissolving some anthocyanins).
Why isn’t the color of the foam more uniform?
This is also the anthocyanins’ doing. The foam can actually have several different colors, predominantly pink, violet, and blue. Anthocyanins can react to the presence of some acids and bases in the media they are dissolved in. Indeed, citric acid is – you guessed it! – acid, and causes anthocyanins to turn pink or even red when the two interact. And, as sodium carbonate is a base, anthocyanins turn blue when they encounter sodium carbonate particles. You can learn more about this phenomenon here in the «Anthocyanins» experiment.