Chemical and physical properties of zinc
Reactions with other elements

Zinc’s chemical and physical properties make it a material suitable for a diverse range of human activities.
How the element was discovered
Humanity has used alloys containing zinc since ancient times. The name of the metal (zincum) can be encountered in the works of Paracelsus, a doctor who practiced medicine in the 16th century. At around the same time, craftsmen in China began forging coins from the metal. Zinc was later discovered in Europe and was soon introduced for widespread use in various spheres.
The first known alloy of zinc, brass, was first used in Cyprus, and then in England, Germany, and other European nations.

The name of the metal comes from the word zincum, the etymology of which is not entirely clear. According to various theories, zincum can be translated from Latin as “white coating,” while the word “zinke” means “tooth” in German. The metal received its modern name in the 20th century.
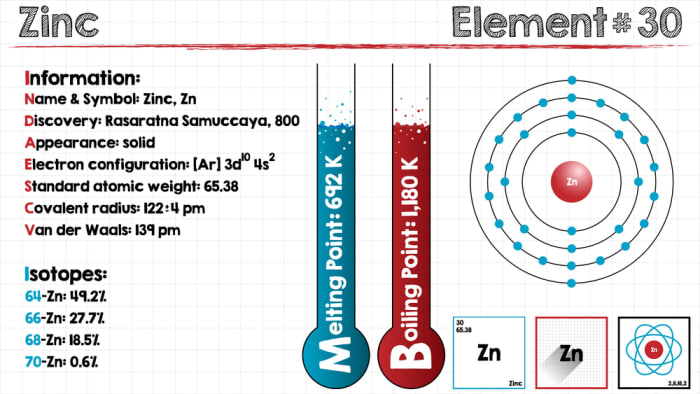
Element characteristics
Zinc is located in group IIB of the periodic table. It has an atomic weight of 65.38 g/mol and an atomic number of 30. The electron configuration of the outermost shell of the atom is 4s². Its constant and only additional oxidation state is +2.
Zinc, like other metals of the platinum group (ruthenium, rhodium, platinum, osmium, palladium, and iridium), is a transition metal and forms complex compounds, in which it acts as a complexing agent. Zinc has five stable naturally-occurring isotopes with masses ranging from 64 to 70. Radioactive ⁶⁵Zn has a half-life of 244 days.
Physical properties of zinc
Characteristics of the element:
- density – 7.13 g/cm³;
- color – bluish-white;
- melting point – 420 °C;
- elasticity and malleability increase when heated to approximately 100 °C;
- boiling point of 906 °C;
- at temperatures above 200 °C, loses its elasticity and becomes a grey powder;
- high heat capacity and heat conductivity;
- good conductor.
Chemical properties of the element
In ordinary conditions, zinc reacts rapidly with air, gradually forming a dull grey zinc oxide coating. Additionally, zinc reacts with halogens, oxygen, chalcogens, alkalis, acids, ammonia and ammonium salts, and even with less active metals. Zinc reacts with both acids and alkalis, making it an amphoteric metal. When reacting with alkalis, the element forms complex compounds known as hydroxo-zincates.
Examples of several reactions with zinc
Zinc ions were discovered based on zinc's reaction with benzoin.

Solutions of sodium thiosulfate, sodium silicate, magnesium chloride, and benzoin in ethyl alcohol are gradually added to the test solution. The released magnesium hydroxide absorbs the complex of zinc and benzoin, and the precipitate glows green when exposed to ultraviolet rays.
When an electric current is passed through a solution containing ions, i.e. through an electrolyte solution, chemical reactions take place on the electrodes, and the degree of the transformation of this reaction is connected with the amount of electricity according to Faraday’s laws of electrolysis. For example, if the solution contains a zinc salt, then the zinc ions precipitate on the cathode (the electrode to which electrons flow) as metallic zinc. This reaction can be regarded as the reduction of zinc ions via electrolysis.
Reduction of the nitro group: a few crystals or drops of nitro compounds are mixed with 0.5 M HCl, and metallic zinc is added. The reaction takes place violently. When the reaction dies down, the mixture is heated until the smell of the nitro compound disappears. The obtained solution is cooled, and several drops are added to an alkaline solution of calcium hypochlorite Са(OСl)₂. In the course of the reaction, the nitro group is reduced to an amino group.

Studies of the metal's behavior in relation to hydrogen chloride dissolved in various organic liquids revealed that hydrogen chloride solution in anhydrous benzene reacts vigorously with zinc, but the reaction halts as soon as the surface is covered with a film of solid zinc chloride, which dissolves poorly in benzene. With the addition of water to dissolve the zinc chloride, the reaction resumes. Disregarding this fact, we may conclude that hydrogen chloride solutions in organic liquids react strongly with zinc. A solution in dry chloroform reacts with zinc just as well as normal aqueous hydrochloric acid, even though the chloroform solution has very low electrical conductivity – lower than the electrical conductivity of the air gap. This demonstrates that the corrosion process is not always connected with the flow of electric current.
Click here for a selection of mesmerizing experiments with zinc.
Zinc compounds
Main compound categories:
- zinc carbides;
- zinc halogenides (fluoride, chloride, bromide, iodide);
- phosphide;
- selenide, sulfite, zinc arsenide;
- thiocyanates, thiosulfates, and cyanides – in aqueous solution in the form of corresponding complexes;
- ammonia complexes – formed by the interaction of zinc with an ammonia solution;
- amphoteric compounds of zinc oxide and hydroxide – used to obtain complexes of hydroxo-zincates.
Zinc compounds are mostly used in organic synthesis. They are also used in industry as components of anti-corrosion agents, alloys, batteries, paints and varnishes. Zinc oxide is also used in medicine due to its anti-inflammatory and antibacterial effects.
You can order a monthly subscription with cool science sets for kids.