Methods of synthesis of nitrous oxide
World-famous compound from the film about street racers
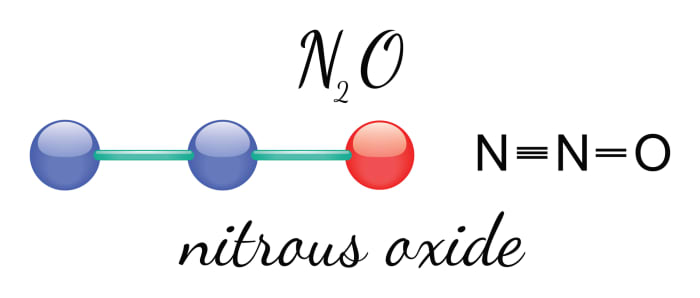
The chemical compound known as nitrous oxide (N20) became world-famous thanks to a popular film about street racers. Do you remember why they used this colorless, non-flammable gas, which has a pleasant sweet smell and taste? That’s right, so they could accelerate before the finish line. But in real life it’s best not to do this, as this substance is dangerous. However, there are many cases where nitrous oxide can be also be used for good purposes.

What ingredients will be required to obtain nitrous oxide (N20)?
- Lyophilized ammonium nitrate NH4NO3 (or sulfamic acid and 73% nitric acid);
- Chemical vessels in which all these experiments will be carried out – flasks, retorts and a burner;
- An electrical heating device which can regulate the temperature.
A precise step-by-step algorithm of the synthesis of nitrous oxide (N20)
All the reactions required for the synthesis of this substance should be carried out exclusively in a specially equipped chemical laboratory, as this process is not safe. Additionally, all safety rules must be followed – the ventilation must be turned on and a fire-extinguisher should be at hand.
The most common method
In the majority of cases nitrous oxide, N2O, is obtained by the thermal decomposition of dry ammonium nitrate, NH4NO3. In the chemical laboratory, nitrous oxide can be obtained by heating dry ammonium nitrate intensely with an electrical device (up to 270 degrees Celsius). In fact, ammonium nitrate NH4NO3 is a well-known substance that is not only used as a fertilizer, but also as an explosive. For this reason, it must be emphasized that the heating temperature should not be above 270 degrees Celsius – otherwise a fatal explosion is guaranteed. Again, in the process of heating lyophilized ammonium nitrate NH4NO3, necessary conditions are ventilation, cooling and collection of the colorless N2O gas in a separate container. It should be clear by now that the greatest danger in this case is the explosive nature of ammonium nitrate. So think once again about whether you really need nitrous oxide or not.

Alternative method
We should also look at another method of obtaining nitrous oxide N20 – thermal treatment by sulfamic acid with 73% nitric acid in the same conditions described in the first case. But bear in mind that this method is used primarily in industry – the material expenses are several times lower, but the danger is much greater (even despite the explosive nature of ammonium nitrate). So you should certainly not attempt to obtain nitrous oxide in this way at home. This is because sulfamic acid can burn the skin and mucous membranes, and nitric acid itself and its fumes are highly toxic: they cause irritation to the upper and lower respiratory tracts, while HNO3 itself damages all the layers of the skin, forming sores that are difficult to treat. Here you'll find a lot of interesting reactions with nitrogen.
Storing the obtained compound
There is another factor that you should take into account – N20 gas only moves to a liquid state under a pressure of at least 40 atmospheres at room temperature. Obviously, this is difficult to achieve at home, to put it mildly.
The easiest way to solve the problem
You shouldn’t risk the life and health of others for a few liters of laughing gas. As nitrous oxide is used as an inhaled anesthetic, it can be purchased from pharmaceutical companies. As N20 is not a narcotic substance, there will not be any problems in buying it.