Sulfuric acid and reactions with it
Chemical properties of sulfuric acid

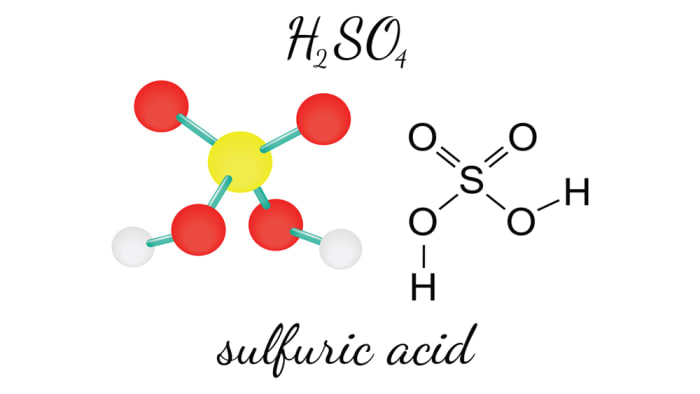
Sulfuric acid is one of the strongest dibasic acids, which has the formula H₂SO₄.
As for its physical properties, sulfuric acid looks like a thick transparent oily liquid with no smell. H₂SO₄ has found wide use in industry, and depending on the concentration of sulfuric acid, the solution has many different properties and spheres of application. Sulfuric acid is used in the following cases:
- treating metals;
- treating ores;
- manufacture of mineral fertilizers;
- chemical synthesis.
History of the discovery of sulfuric acid
Sulfuric acid has been known to humans since ancient times, and it was mainly found in nature, for example in volcanic lakes.

In the 9th century, the alchemist from Persia Muhammad Ar-Razzi obtained a solution of sulfuric acid by the method of burning copper and iron sulfate.
But the method of the Persian alchemist was improved four centuries later by the European scientist Albert Magnus.
The modern industrial (contact) method of obtaining sulfuric acid involves oxidizing sulfur dioxide, a gas which forms on the combustion of sulfur or sulfur pyrite. Sulfur trioxide forms, and interacts with water.
Contact sulfuric acid has a concentration from 92 to 94 percent:
2SO₂ + O₂ = 2SO₂;
H₂O + SO₃ = H₂SO₄.
Physical and physico-chemical properties of sulfuric acid
H₂SO₄ mixes with water and SO₃ in all proportions.
In aqueous solutions of H₂SO₄ hydrates form of the type H₂SO₄∙nH₂O
The boiling point of sulfuric acid depends on the level of concentration of the solution and reaches its maximum at a concentration of over 98 %.
The best-known compound in industry is the caustic compound oleum, which is a solution of SO₃ in sulfuric acid.
When the concentration of sulfur trioxide in oleum is higher, the boiling point drops.
Chemical properties of sulfuric acid
When heated, concentrated sulfuric acid is a strong oxidizer, which can oxidize many metals, with the exception of:
- Gold (Au);
- Platinum (Pt);
- Iridium (Ir);
- Rhodium (Rh);
- Tantalum (Та).
When concentrated sulfuric acid oxidizes metals, it can reduce to H₂S, S and SO₂.
Active metal:
8 Al + 15H₂SO₄(conc.)→4Al₂(SO₄)₃ + 12H₂O + 3H₂S
Metal of medium activity:
2Cr + 4 H2SO4(conc.)→ Cr2(SO4)3 + 4 H2O + S
Metal of low activity
2Bi + 6H₂SO₄(conc.)→ Bi₂(SO₄)₃ + 6H₂O + 3SO₂
With cold concentrated sulfuric acid, such metals as iron and aluminum do not react, as they are covered with an oxide film. This process is called passivation.
Reaction of sulfuric acid and H₂O
When H₂SO₄ is mixed with water an exothermic process takes place, i.e. a large amount of heat is released and the solution may even boil. When conducting chemical experiments, one must always add sulfuric acid to water, not the other way around.
Sulfuric acid is a strong dehydrating substance, and concentrated sulfuric acid forces water out of various compounds. It is often used as a drying agent.
Reaction of sulfuric acid and sugar
The affinity of sulfuric acid for water can be demonstrated by a classic experiment, by mixing concentrated and sugar, which is an organic compound – a carbohydrate. In order to remove water from a substance, sulfuric acid is capable of destroying molecules.
To conduct the experiment, add a few drops of water to sugar and mix, then carefully add sulfuric acid. After a short period, one can observe a violent reaction, with the formation of carbon and the release of gases, sulfur dioxide and carbon dioxide.
Sulfuric acid and sugar cube:
Remember that working with sulfuric acid is very dangerous without observing safety rules, as sulfuric acid is a caustic substance that can instantly leave serious burns on the skin.
Here you’ll find safe chemical experiments with sugar
Reaction of sulfuric acid and zinc
This reaction is quite popular, and are one of the most widespread laboratory methods for obtaining hydrogen: if you add zinc granules to diluted sulfuric acid, the metal will dissolve with the release of gas:
Zn + H₂SO₄ → ZnSO₄ + H₂
Diluted sulfuric acid reacts with metals which are to the left of hydrogen in the row of activity, according to the general scheme:
Ме + H₂SO₄(diluted) → salt + H₂↑
Reaction of sulfuric acid and barium
The qualitative reaction to sulfuric acid and its salt is the reaction with barium ions. This reaction is widely used in quantitative analysis, in particular in gravimetry.
H₂SO₄ + BaCl₂ → BaSO₄ + 2HCl
ZnSO₄ + BaCl₂ → BaSO₄ + ZnCl₂
Warning! Don’t try to repeat these experiments without a professional supervision!