Chemical ink eraser
An inscription written in pen is erased by potassium permanganate


Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The drawing disappeared only partially. What should I do?
There are still some pen traces on the paper? Probably, potassium permanganate did not have enough time to erase the drawing completely. Dry the paper properly. Repeat instructions steps 8-10. Make sure that you wait 15 minutes after completing p. 8!
There are purple and (or) brown stains left on the paper. How to clean them?
If there are some stains left on the paper, you should use more Na2S2O5 solution. Purple stains are potassium permanganate KMnO4, while brown manganese dioxide MnO2 (see the answer for question «What do we need a reductant for?»).
Add a few drops of Na2S2O5 solution onto the stains. Wait 2-3 minutes and dry them with filter paper. If you ran out of filter paper, you can use a regular paper towel.
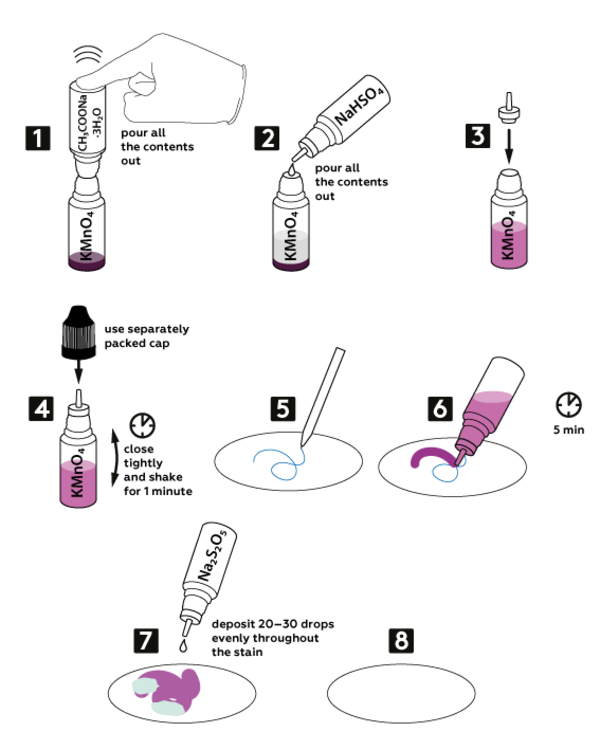
Step-by-step instructions
-
Pour out all the contents of the vial with sodium acetate CH3COONa*3H2O (1.4 g) into the vial with dry potassium permanganate KMnO4 (0.2 g).
-
Add all 3М sodium bisulfate NaHSO4 solution (5 mL) into the vial with the mixture of potassium permanganate and sodium acetate.
-
Now, take a new nozzle from the set and insert it into the vial with the obtained mixture, as shown.
-
Close the vial with a black cap and shake it for 1 min.
-
Write or draw something on paper with the pen provided — not all the inks can be equally well erased with this method.
-
Drop by drop deposit the solution obtained in step 6 onto your drawing made with the pen. Wait 5 min.
-
Evenly distribute 20–30 drops of 0.5M sodium pyrosulfite Na2S2O5 solution over the drawing.
-
Your drawing has disappeared!
Expected result
Potassium permanganate removes pen marks from paper.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Why does potassium permanganate decolorize ink?
Potassium permanganate KMnO4 is a strong oxidizing agent. It means that permanganate reacts with various substances and takes their electrons away. Dyes are specific substances, which are responsible for the ink color. Usually, dyes are complex organic compounds. Thanks to their specific structure, dyes provide the ink with bright and deep color, even when added in very small amounts. Potassium permanganate oxidizes dyes while changing their structure. That is why dyes, and ink at the same time, decolorize.
More details:
Let us start with the fact that permanganate oxidizes double bonds in dyes molecules. This reaction destroys the conjugated system:
R2C=CR2′ + KMnO4 + H2O + H+ → R2COH–CR2′OH + MnO2↓ + K+
What do we need a reductant for?
We have successfully decolorized the ink with potassium permanganate. However, some brown manganese dioxide MnO2 is formed in this reaction. It is very poorly soluble in water, so it is impossible to wash it off from paper. That is why we need a reductant – disodium disulfite Na2S2O5. Disulfite reduces brown MnO2 to colorless Mn2+. The reaction is as follows:
MnO2 + Na2S2O5 + NaHSO4 → MnSO4 + Na2SO4 + H2O
More details:
MnO2 precipitate is very hard to remove from paper. However, it is not the only reason why we use disulfite. Paper has partially absorbed excess of purple KMnO4 solution we used in the first step. Disodium disulfite reduces purple permanganate to colorless Mn2+ salts:
KMnO4 + Na2S2O5 + NaHSO4 → MnSO4 + Na2SO4 + H2O
What do we use sodium acetate for?
To be honest, we need aqueous solution of acetic acid for this experiment. Unfortunately, European safety standards for children science kits prohibit including acetic acid (or its solution in water). Luckily, this is all about chemistry, so there is no problem in obtaining acetic acid aqueous solution during our experiment.
When dissolved in water, NaHSO4 releases H+ and makes the solution quite acidic. Sodium acetate CH3COONa in this acidic medium forms acetic acid:
CH3COONa + H+ → CH3COOH + Na+
Acetic acid is an organic compound by nature, and it is also highly soluble in water. In turn, ink consists mostly of organic compounds that are poorly soluble in water. Acetic acid helps these compounds to migrate from paper into aqueous solution where the reaction with an oxidizer takes place.
Without acetic acid, there would be very few dye molecules in the solution. Then, the majority of them (absorbed into paper) would not decolorize.
Reactions proceed much faster if reagents are mixed thoroughly. When substances are separated (or mixed not well enough), they can exchange molecules only through the surface they are separated with. In such cases reactions proceed very slowly.
Why does the ink become colorless during the oxidation?
Let us talk a little about the nature of color. It is evident that we see almost nothing in the dark. All the things seem more or less black. Therefore, we need a light source (sunlight, electric lamp or flashlight) to distinguish colors. A light source sends an immense amount of light particles called photons. Every photon possesses certain energy. A light beam may be compared with a traffic stream on a big and busy road where numerous cars move in the same direction. However, photons move much faster than cars, and there are incredibly more of them. Actually, one cannot even imagine this amount!
Some of these photons are absorbed by the material while some are reflected. Most photons pass through transparent surfaces such as glass. Human eye catches the reflected part of light beam (photon stream). Our brain interprets it as a color.
It is the structure of a particular substance that determines how many and which photons (depending on their energy) would be absorbed. Usually, dyes are organic compounds of complex structure. They are spread equally throughout the colored material. Dyes actively and selectively absorb photons of certain energy. That is why in case of dyes we recognize the reflected photons as bright and deep color.
The bond structure of dyes often includes a long conjugated system of alternating single and double bonds. Such electronic structure allows the substance to absorb photons actively and selectively. Consequently, it makes dyes so bright.
An oxidizing agent promptly destroys the conjugated system of dye. Substance starts to absorb less and less photons and eventually becomes colorless. At that point, we can only see white paper, which absorbs light poorly. White color of an object means that it mostly reflects the photon stream.
What else can we decolorize ink with besides KMnO4?
There are two different ways to complete this task.
The first method has already been demonstrated in our experiment. We have oxidized dyes in the ink with a strong oxidizing agent: permanganate. One can try other oxidizing agents. For example, fresh hydrogen peroxide solution (first aid kit disinfectant) should be able to decolorize ink. However, we do not recommend you to try experiments like that. Most strong oxidizers are quite dangerous substances, which should be handled with knowledge and care.
The other method is to dissolve the ink or the dye from it. You can use nail polish remover for this purpose. It usually contains a mixture of organic solvents such as acetone and isopropanol. Using a cotton swab, carefully apply a little bit of nail polish remover on a paper with ink. Then wipe it off with another cotton swab. Repeat this procedure several times. Be careful! Nail polish remover is toxic and flammable. Do not breathe it in, avoid skin contact, and wear splash goggles to protect eyes.
Why is KMnO4 a strong oxidizing agent?
Manganese atom in KMnO4 has a significant positive charge: Mn+7. It means that a maximum amount of electrons has been removed from its outer electronic shell. The latter participates in various chemical reactions. Obviously, Mn+7 readily oxidizes other substances to obtain electrons from them. The reaction produces much more stable Mn+2 or Mn+4 (which precipitates in form of MnO2).
Generally, the power of oxidizer (or oxidizing ability) is determined by its tendency to possess more electrons than they already have (in other words, to take electrons from other atoms). Strong oxidizers usually feature an atom with a significant positive charge (such as Mn+7 in our case).
That’s interesting!
What makes ink colored?
Dyes are responsible for ink color. We have already mentioned that normally dyes are complex organic compounds, which have characteristic bright and deep color. One needs only a tiny amount of dye to color a material. The following substances may be found in an ordinary blue ink:
-
Fuchsine (or aniline red): has magenta-red color when dissolved in water. One of the pioneer synthetic dyes, it was first obtained in 1856. Its derivatives are used in inks.
-
Indigo: blue-purple dye used for jean color.
-
Methyl violet: surprisingly, it is violet!
-
Indigo carmine: blue dye well soluble in water.
More details:
It is important to note that many dyes are pH-sensitive. They can change color when the concentration of H+ ions shifts or when OH– ions appear (in basic solutions). Such compounds are widely used as pH-indicators in analytic and industrial chemistry. Most of the reactions responsible for an indicator dye color change are acid-base processes. Usually, indicators are complex organic salts.
What is ink made from? Can we erase any ink with the chemical eraser?
Ink composition may be quite complex and differs depending on its manufacturing process. Moreover, many companies keep their ink formula in secret! Generally, ink contains a solution of several components in an appropriate solvent.
One can separate ink components into groups. The first group is solvents such as distilled water, glycerine, and ethanol. The second is dyes: indigo, fuchsine, methyl violet, indigo carmine, iron (II) sulfate, etc. And the third is modifiers. The latter are special substances, which influence ink viscosity and drying rate or provide ink with specific smell. Examples of modifiers are polyatomic alcohols, oxalic acid, latex, and perfume.
We have listed only a few substances that may be found in ink. Certainly, there are many more of them! Besides, companies vary proportions of these substances in every single type of ink. Some inks are very difficult to remove from paper, and some cannot be decolorized at all. We have tested a number of pens and selected the one suitable for our experiment. Markings made with this pen can be erased smoothly with our chemical eraser. Enjoy!