Carbon filter
Purify water with a DIY filter!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Simply pour the excess of activated carbon back into the bottle and continue with your experiment.
Absolutely! Feel free to continue the experiment. If you prefer, you can use a wooden stick to dislodge these particles.
Make sure you inserted two cotton cylinders: one below and one above the carbon layer. It’s also possible the carbon wasn’t pressed down properly. Repeat the experiment, paying particular attention to these details.
It’s also possible that the pieces of carbon are too big. If this seems to be the case, pour your carbon into a clean plastic cup and use a wooden stick to grind it into finer pieces. You can also do this in the filter, but only after removing the upper cotton cylinder.
If water seems to be moving through your filter too slowly or not at all, there is probably too much air in the carbon section of the filter.
First, press down on the upper cylinder a bit with a wooden stick. Then, squeeze the filter walls with your fingers to force any air out. This action should somewhat resemble squeezing toothpaste from a tube. Just be careful not to spill the solution you’re filtering. This should work!
If this still doesn’t help, try repeating the experiment.
Actually, nothing! This grey substance is none other than some activated carbon particles. Some of them are very tiny and just passed through the cotton cylinders while the organic contaminant (methylene blue) was filtered out.
This happens in everyday household carbon filters as well. Normally, you need to cycle water through such filters 2-3 times before using them to filter water you intend to drink!
Filter a small fraction of the solution, wash the vial, and try to filter some more – the water will eventually emerge clear and free of carbon pieces.
Yes, of course! You may get a slightly different result (for example, the solution may not be completely colorless, but it will be much lighter). Don't be afraid to experiment!
That's a very good question! You can try, of course. If you have activated carbon at home, grind it and use it for the experiment. The difference between coal and activated carbon lies more in their adsorption properties. Grind the coal, assemble the filter, and pass a solution of methylene blue through it. Compare your results to what happened when you used an activated carbon filter. Write to us and let us know about the results!
Despite filtering out most of the methylene blue, our filter is not yet effective enough, so do not drink the filtered water!
Step-by-step instructions
Assemble a charcoal filter. To make it more effective, compress the activated charcoal a bit using cotton cylinders and a wooden stick.
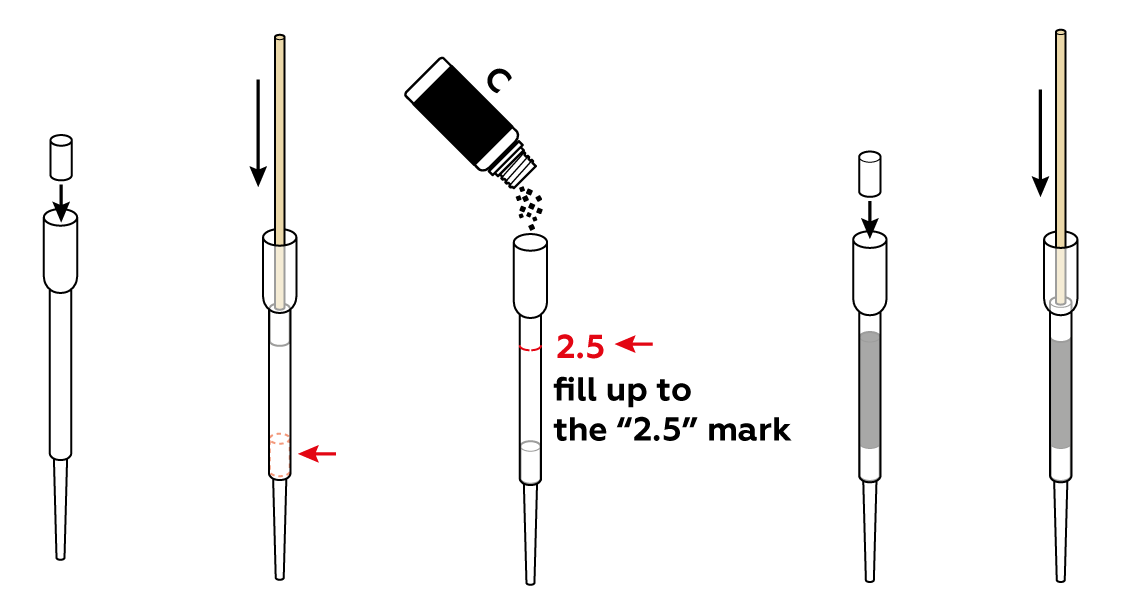
Set up the test stand.

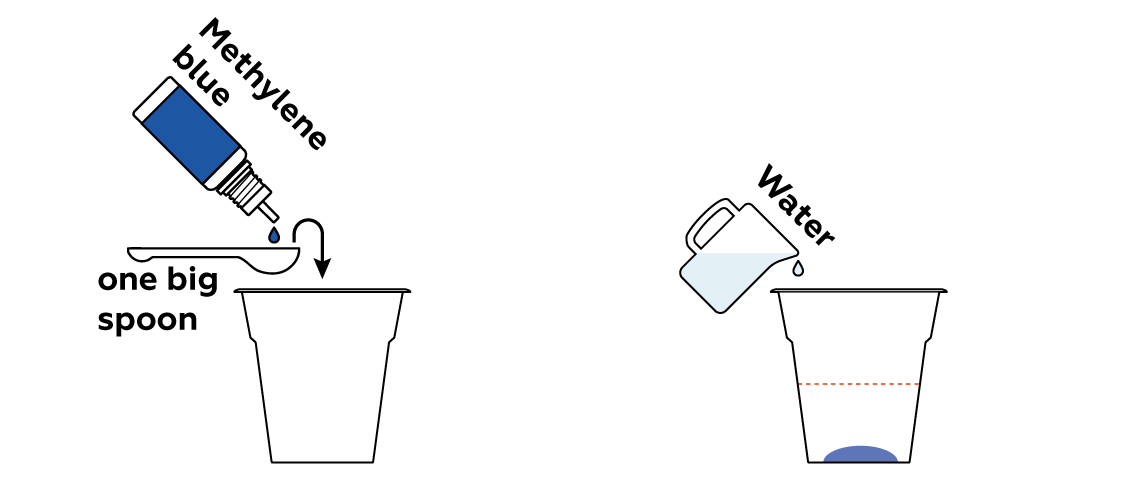
Make an organic pollutant solution using water and methylene blue. Methylene blue is widely applied in industry to test filters’ abilities to effectively purify drinking water.
The filter adsorbs the organic pollutant, and purified water fills the vial.
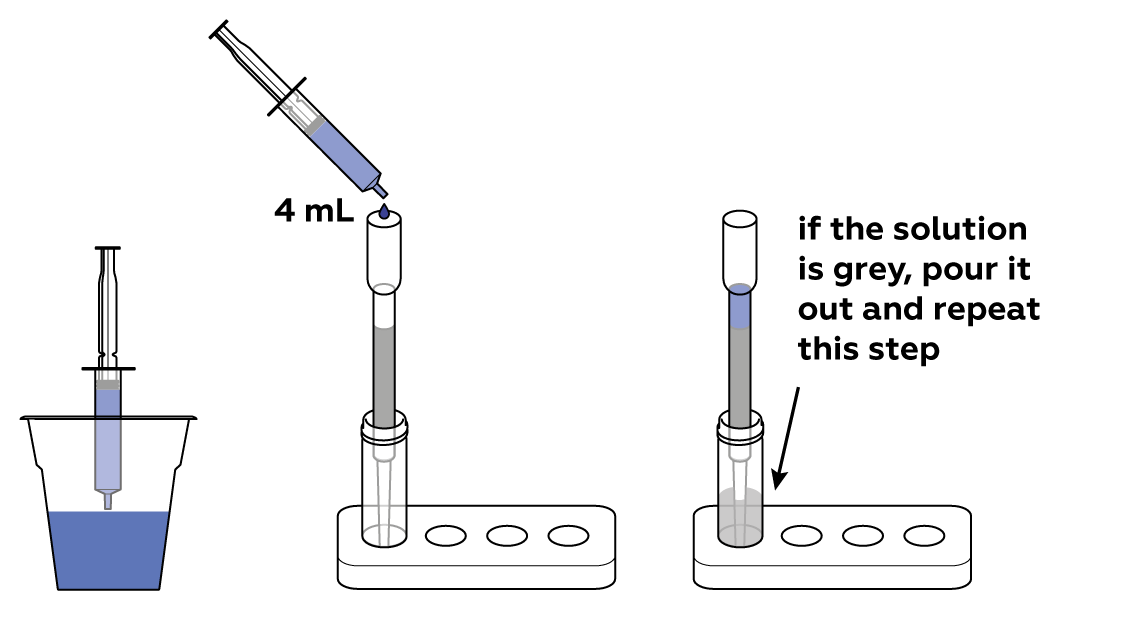
Wash the vial to repeat the experiment.
Expected result
Coal sorbs methylene blue molecules from water leaving the water clear and colorless.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink and wash with an excess of water.
Scientific description

Pesticides used to treat crop fields, chemical manufacturing residue, and other manmade pollutants first seep into the soil, then trickle into rivers, lakes and into our drinking water. Since many of these substances are harmful, our water must be purified before we can drink it.
Among these pollutants there are rather big organic molecules . Methylene blue
has a similar structure and gives water a strong color, making it a good substance for testing filters.
Particles with such a structure readily “stick” to the charcoal surface (adsorbing to it). Activated carbon is made of charcoal, but unlike ordinary charcoal it has a much larger surface area due to its huge amount of cracks and pores. This is why so many pollutant molecules “stick” to it, while water molecules pass through easily.
The carbon filter deals with many organic pollutants well, but can’t do much with some other harmful substances, like heavy metal ions . To get rid of these, ion-exchange resins are used, as we do in another experiment in this set.
This experiment is possible due to a very important process called sorption. This process can be of either a chemical, physical, or mechanical nature, but its working principle is always the same: one particular substance “soaks up” other substances, removing them from a solution or reaction mixture. In this case, the process of sorption takes place on the surface of the activated carbon. This surface sorption is called adsorption, while activated carbon is the adsorbent that “soaks up” methylene blue from the solution.







Most bacteria, in turn, can be eliminated by boiling the water or using some special antibacterial additives. On a large scale, water is disinfected via ultraviolet treatments or by the addition of ozone, chlorine, or other effective oxidizing reagents.
That’s interesting!
From the moment our ancestors started living in large groups, people have been relentlessly polluting their surroundings. At first, we created waste that nature could deal with relatively easily: leftover food, wood chips, and natural fabrics (leather, fur, and plant fibers). However, once humans learned to extract metals from ore and produce synthetic materials, the environmental damage caused by thoughtlessly-scattered waste rose to catastrophic proportions.
A wide range of compounds are water-soluble, and not all of them are as harmless as salt or sugar. Some dissolved impurities betray their presence with a telltale color, taste, or odor. Characteristics of water indicating the presence of impurities are called organoleptic properties. Of course, few would drink malodorous or muddy water.
Unfortunately, it is often impossible to detect hazardous substances in harmful concentrations without using special instruments. We use water filters to rid our drinking water of these impurities.
A key component in many filters is a sorption material, or an adsorbent. This is a solid compound, insoluble in water, that is capable of capturing and adsorbing other substances from water or air.
Let’s digress for a moment. Many of you know that ants can carry 50-100 times their own body weight. For a person, that would be equal to carrying a fully-grown elephant! In a certain way, an adsorbent is a very strong inanimate “ant”.
How does an adsorbent work? The total mass of the substances it adsorbs is much greater than its own mass. Moreover, if the adsorbent is efficient, it is difficult to retrieve the adsorbed substances from the adsorbent; they are firmly trapped inside the adsorbent and unable to escape back into the already-filtered water or air.
One of the most popular adsorbents is activated carbon, which is produced by heating raw wood under pressure created by water vapor. In order to make purification more efficient, nut husks (usually coconut) are sometimes used instead of wood. Importantly, adsorbent particles must be porous and very small: the more numerous the pores, the larger the net surface area of the material, which, in turn, facilitates adsorption.
We use methylene blue dye to analyze the quality of activated carbon. This dye is relatively harmless (unless you spill it on a clean shirt!) and is similar in molecular structure to many organic pollutants that can be found in drinking water, such as pesticides. The presence or absence of this dye (i.e. whether or not it has been successfully adsorbed) is visible to the naked eye. The more efficiently carbon or a carbon-based filter removes methylene blue from water, the more efficiently it will remove many pollutants.
Water must be carefully analyzed prior to purification. The method of purification is chosen depending on which pollutants are most prevalent.
-
Adsorption: an adsorbent (usually coconut coal or charcoal) traps and removes organic impurities from water.
-
Ion exchange: special resins interact with a solution to trap ions of heavy metals.
-
Water chlorination is a method used to kill pathogenic bacteria in the water.
-
Mechanical filters can purify water of sand, silt, mud, rust, and other insoluble particles.
-
Electrolysis (water desalting) is driven by an electrical current that removes various kinds of impurities like metal ions. As a result, these impurities are expelled as gases or precipitates that can be removed easily using a mechanical filter (this technique is usually used to remove metallic impurities from water).
Nevertheless, none of these methods can purify water of all impurities on their own. In order to properly purify water, we use complex multi-level filter systems that employ several of these methods at once.
Dozens of experiments you can do at home
One of the most exciting and ambitious home-chemistry educational projects
The Royal Society of Chemistry
