Don't blink!
A solution is suddenly plunged into darkness!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Observe safety precautions when working with boiling water.
- Don't consume any of the components.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Sure! You have several options: noodles, raw potato, or packaged starch from the grocery store. If you opt to use a potato, grate some raw potato into a disposable cup, then fill the cup halfway with boiling water. In about 10–15 minutes, the potato starch will dissolve in the water and you can use it as your source of starch for the experiment.
Both temperature and reagent concentrations affect the reaction rate. The water you used may have been too warm. Use colder water or add more Na2S2O5 solution to reduce the reaction rate and increase the time it takes for the solution to darken.
It’s likely that the water is too cold. Repeat the experiment using room temperature water. You can also try adding less Na2S2O5 solution – use 6 or 7 drops instead of 10. Try different options and see which works best!
You may have added too much Na2S2O5 solution, or perhaps the starch pellets didn’t dissolve very well. Repeat the experiment, measuring the amount of reagents carefully and letting the pellets sit in the boiling water for longer.
This can happen if the water poured over the starch pellets is not hot enough and not enough starch dissolves. Repeat the experiment, using boiling water to dissolve the starch.
Step-by-step instructions
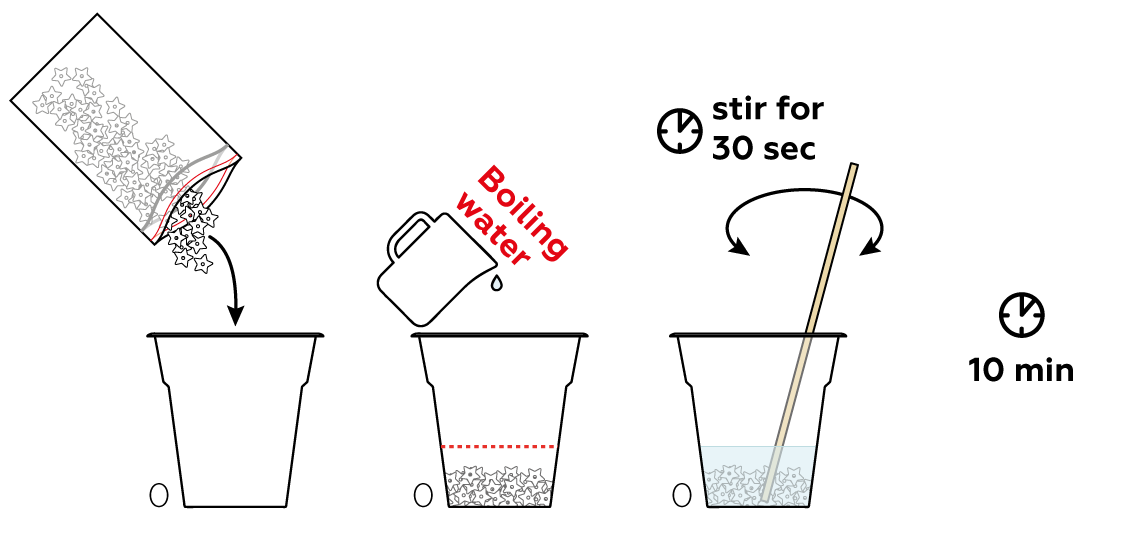
First, you need to prepare a starch solution. Starch readily seeps from the pellets provided into hot water.
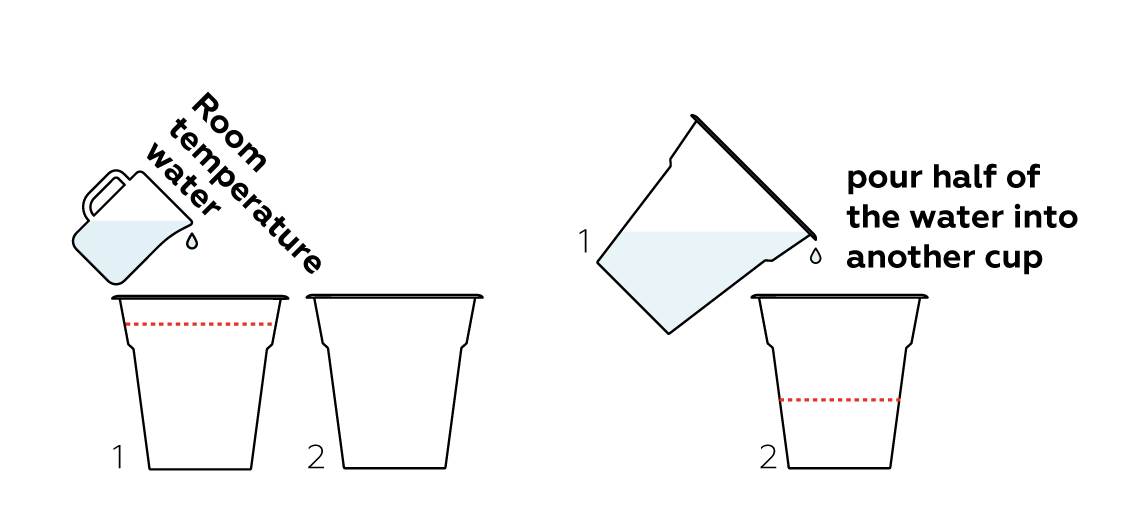
While your starch solution is brewing, fill two cups halfway full with room temperature water.
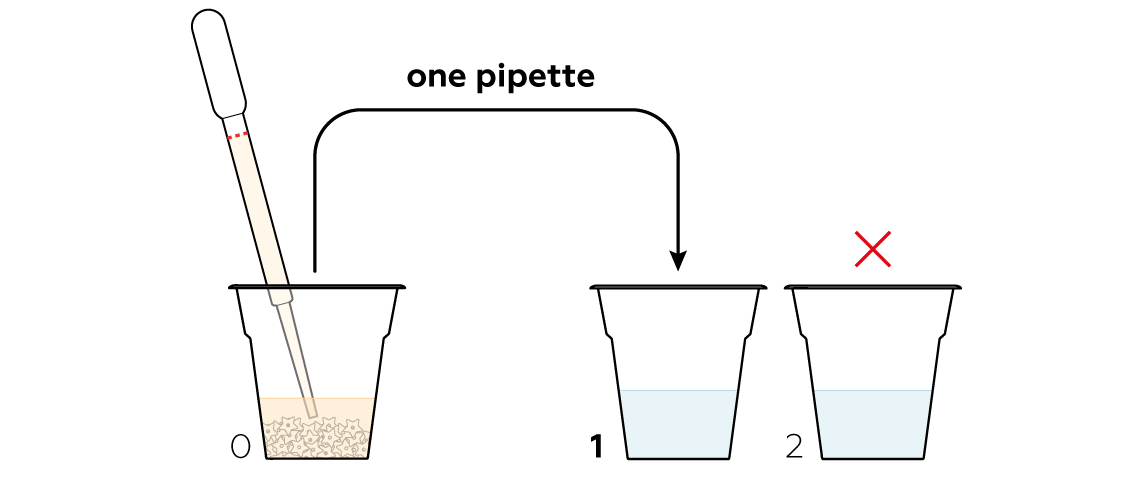
Starch molecules are really sensitive to iodine molecules I2: once they meet, starch immediately turns dark blue.
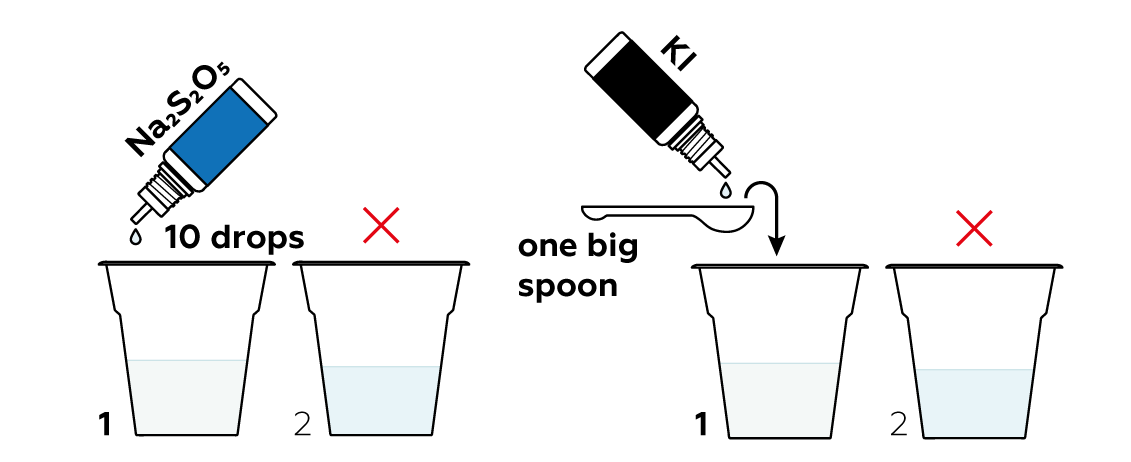
KI yields iodide particles I-. Though they may sound similar, these aren’t iodine molecules I2, and won't make starch blue. Rather, iodide particles I- have extra electrons e- and aren't really keen to bind and form I2 molecules. Moreover, the S2O52- from Na2S2O5 is quite willing to give any emerging I2 molecules extra electrons and quickly turn them back into I- particles. Thus, the Na2S2O5, KI, and starch solutions together in a cup won't do anything notable just yet.
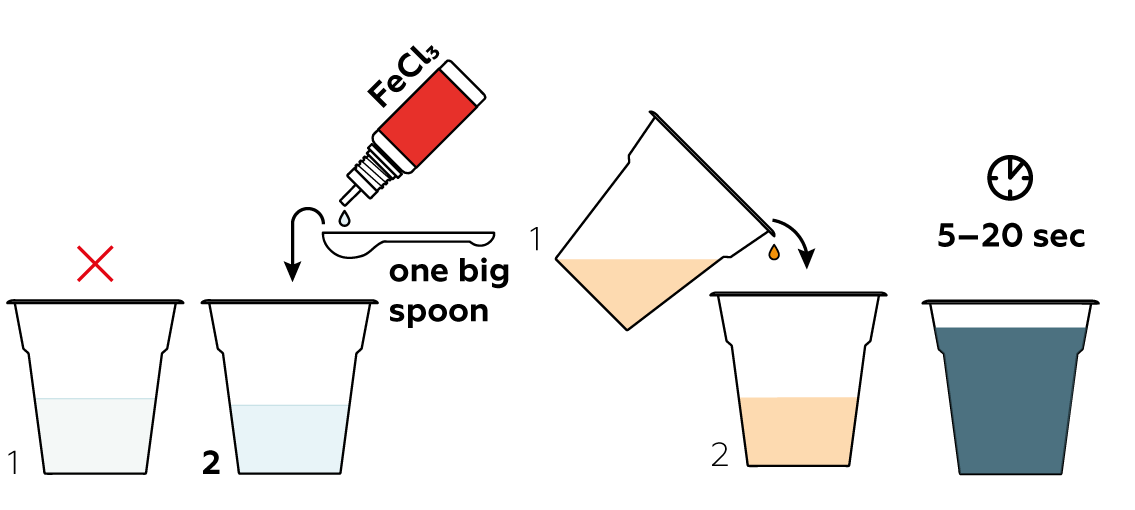
Add some FeCl3 to the other cup. Fe3+ is really eager to grab electrons from I-, helping them combine to form iodine molecules I2. Mix the liquids and don't blink!
Disposal
Dispose of the reagents and solid waste together with household garbage. Pour solutions down the sink and wash with an excess of water.
Scientific description
Initially, not much seems to be going on, but a whole host of processes are churning away under the hood. Fe3+ 



is quickly turning them back into I\-


are fighting for iodine's attention, but S2O52\-
takes the lead.But this state of affairs can’t last forever—both Fe3+

are slowly being used up in the process. However, there's much more Fe3+

. Once the S2O52\-
is used up, Fe3+





