Distillation
Purify water by evaporating and recondensing it!

Safety
- Carefully review the safety advice on the back of the box cover before starting the experiment.
- Put on protective eyewear. Conduct the experiment on the plastic tray.
- Keep a bowl of water nearby during the experiment.
- Make sure the setup parts are connected securely. Gaps between them may lead to steam burns.
- Do not extinguish the solid fuel. Let it burn down completely.
FAQ and troubleshooting
It’s been 20 minutes, but there’s still no liquid gathered in the test tube.
Intense heating is required for successful distillation process. You’ll have to wait a little longer if you use a cold drink.
Liquid has sucked back into the flask. Why?
Upon heating, volume of air in the flask increases. However, when you extinguished the candles, air in the flask contracted, and the pressure dropped in there. As a result, the liquid sucked back into the flask to equalize pressure in the whole system. Repeat the experiment starting from the step 4 in order to obtain an odorous essence.
This might have also happened in case you removed the tubing from the test tube and placed it directly into the beaker filled with water— then almost all the liquid would have sucked back into the flask.
Step-by-step instructions
A variety of substances can be distilled. You can try apple juice, then try orange juice or even cola.
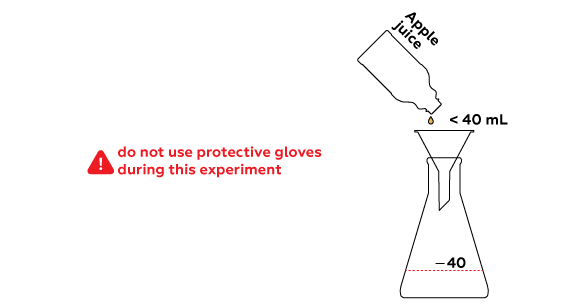
The chosen liquid will be heated in a flask; vapors of the distilled substance will pass to the test tube through the silicon tube.
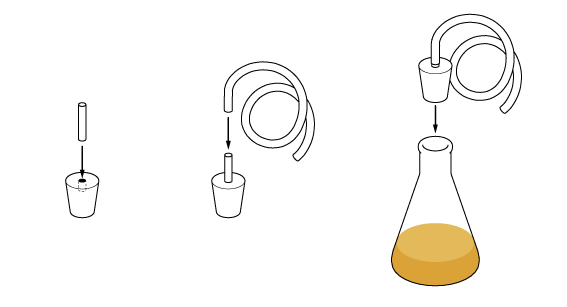
To turn the vapors back to their liquid form we’ll need to cool them. To do this, place the test tube in some cold water.

We need a lot of heat for this distillation, so prepare three candles.
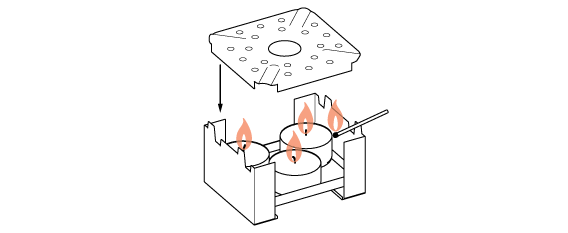
Liquid inside the flask heats up and begins to rapidly evaporate. However, not all of its contents evaporate at the same rate. Odorous substances “fly” more readily than water or colorants (pigments or dyes).
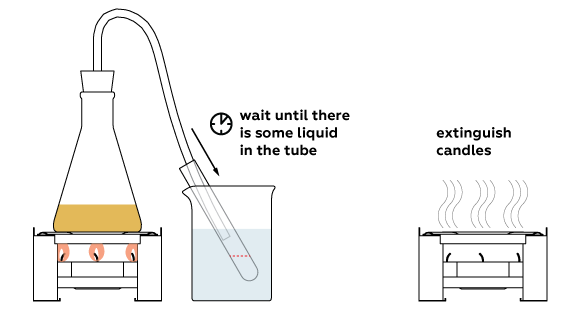
Liquid becomes colorless: all the colorants stayed in the flask.
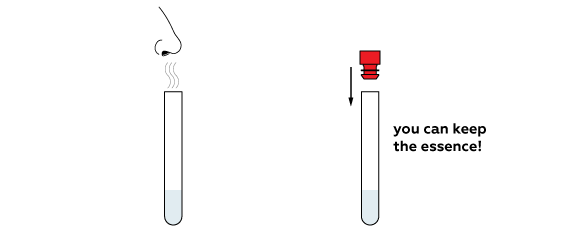
Disposal
Keep the vial of distilled water. Pour the leftover water down the sink. Wash away with an excess of water.
Scientific description
How does the liquid travel from the flask into the test tube?
When heated, any liquid starts to gradually evaporate, or passes to a gaseous state. The process is reversible, and if cooled, vapors turn back to a liquid state. This is what happens in our experiment. Liquid is evaporated from the hot flask, and then vapors travel until they transform into liquid in the cold test tube.
As it would happen with any other liquid, upon heating molecules accelerate. Interestingly, some of these molecules gain such a high speed that they can escape from the liquid and fly into the air. When temperature is kept high enough, the air above the liquid becomes saturated with molecules that were in the liquid state before. Thus, at the beginning of our experiment, molecules that were part of the mixture fill in the air-first in the flask, then in the tube, and finally in the test tube. When these “hot” molecules hit a cold surface, they lose their speed and accumulate on the walls, forming small droplets. These droplets gradually increase in size and finally drain into the test tube where we collect the liquid product. This is exactly what happens in our experiment: the test tube is cooled with cold water, so the molecules that fly out of the flask are collected into a liquid phase in the test tube.
Note that this process called distillation is typical for many liquids. For instance, in the same manner would behave pure water, alcohol, juice, and even broth. Part of their molecules would leave the heated flask and fly towards the cooled test tube. Thus, using heat, we transfer molecules from the flask into the test tube, similarly to moving balls from one basket to another.
Is the essence drinkable?
No, it isn’t!
Follow up
What other liquids can be distilled?
You can try to distill any juice: orange, cherry, pear, pineapple etc. in some cases you will get strong scented essences in others not. Study different juices!
Try to distill lemonades of the most well known brands!
It is strongly forbidden to distill some liquids in the way described in the experiment. Do not distill nail polish removers or other flammable solutions or mixtures under any circumstances.
That’s interesting!
What is distillation?
Distillation (from the Latin distillatio, meaning “trickling”) is the process of transporting fluid in its vaporized state from one vessel to another. What is the meaning of this process? Essentially, some liquids are easier to evaporate than other – because they are more volatile. Thus, in case of a two-component liquid mixture distillation, a relatively more volatile liquid will evaporate faster than the less volatile one.
Still, in many cases distillation does not allow us to separate components of a liquid mixture. One example is a mixture consisting of many components. If there are components with similar volatility in such a mixture, they will evaporate at the same rate. For instance, it happens with fruit juice distillation: esters responsible for the juice smell evaporate all together, so the smell of the corresponding condensate (an essence) is very similar to the smell of an original juice, but is much stronger.
Having been one of the basic processes in alchemy, distillation was thought to be a kind of magical ritual of obtaining the spirit of a liquid, in other words – its essence. You can learn more about distillation in alchemy from the video below:
Distillation is an important process in science and chemical industry. For instance, this process allows separation of petroleum composing fractions, which then serve a variety of needs in industrial production: from fuel and lubricants to protective layer materials for roofs and roads. In particular, the lightest fractions of petroleum (naphtha and gasoline) are mainly used as a starting material for chemical synthesis, as a fuel for car combustible engines, and as an inexpensive solvent in industry. Heavier kerosene and diesel fractions found their use as a fuel for diesel and jet engines. The remaining dense and heavy residue (heating oils) is used as boilers fuel and for lubricants production. Finally, after the removal of all fluids, the solids (asphalt) are used in roof coatings and road construction.
In chemical synthesis, distillation helps to remove organic solvent impurities, as well as to obtain new liquid substances in their pure form. This process was discovered in ancient Egypt by alchemists who were predecessors of chemists on their path of experimenting with substances.
How did alchemy aid the development of science and industry?
Alchemists were ancient experimenters who worked with substances and studied their transformations. Despite the fact that their explanations for observed phenomena were ridiculous and that many, if not most, experiments were meaningless, they left behind a vast heritage. Alchemists developed a variety of approaches for analyzing substances, as well as a comprehensive toolkit still used by chemists. For instance, a significant contribution to medieval metallurgy is distinguished among other alchemists’ achievements. They discovered arsenic As and antimony Sb used in various alloys manufacturing, and they also developed a new design of smelting furnaces.
It was alchemists who for the first time ever isolated and described such substances as acetic, nitric, sulfuric, and hydrochloric acids, and ethanol. Interestingly, the name for a a nitric and hydrochloric acids mixture – aqua regia, meaning “regal water” or “regal liquid” – came from the fact (also discovered by alchemists) that this mixture is capable of dissolving gold, the king of metals. Finally, study and production of gunpowder was also an important contribution of alchemy to the development of military science.
Yet, it is considered that the main achievement in alchemy is emergence of chemistry itself. Nevertheless, by the 18th century, alchemy has smoothly evolved into the establishment of chemistry as an exact experimental science.