Ions
Students will zoom inside a sodium chloride crystal and explore its "atoms." They will see that the sodium atoms are missing one electron, while the chlorine atoms have one additional electron. In this way, students will explore the concept of ions.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

A long time ago, scientists noticed that water that was free of impurities did not conduct an electric current at all.
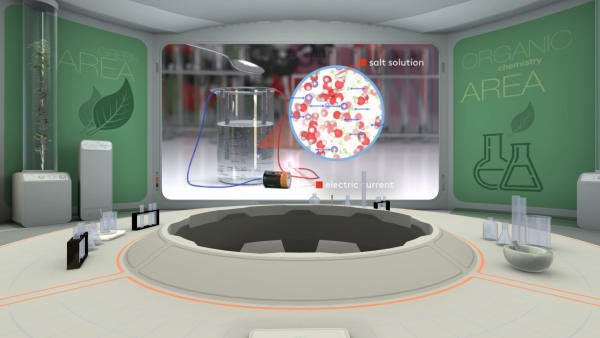
But if salt is poured into the water, the solution will become a conductor. Look, the light is on!

But why does an aqueous solution of sodium chloride conduct electricity?
To answer this question, let's take a small grain of table salt and look inside it.

So right now you are as small as an atom and you are flying inside a crystal. You have probably already noticed that there are particles of two kinds here - smaller…… and bigger ones.
These particles look like atoms. But let's take a closer look at these particles.

We start with one that is smaller in size.

It looks like a normal atom.
It has a nucleus and an electron shell.

And it consists of 10 electrons……. 12 neutrons…….. and 11 protons.
So, as it contains 11 protons it seems to be a sodium atom.
But look, there are only 10 electrons in this particle!
You might say: Well, what's wrong with that? Let it be ten electrons, what's the problem?

Let's try to build a sodium atom. Protons and neutrons form the nucleus.
And as we have 11 protons, so we need 11 electrons to form a sodium atom. But we have only ten! In our particle, there is one electron less than in the sodium atom and the particle as a whole will have a positive charge plus one.

Let's add one more electron.
Now it is a sodium atom.
It is much bigger than our particle.

Remove the electron from the sodium atom and look again at our particle. Our particle is much smaller than the sodium atom. And it has a positive charge.
We call this particle an ion.
And we have a special symbol for this positive sodium ion: sodium plus. But who could take an electron from a sodium atom? Let's find this thief.

We go back to our salt crystal.
Now let's look carefully at the bigger particles.

Choose this one, for example.

It also has a nucleus and an electron shell. It consists of 18 electrons…… 18 neutrons……. and 17 protons.

So, it seems to be chlorine.
But if we build a chlorine atom from these particles, then one extra electron will remain.

We attach this electron to the chlorine atom and get our particle.

In this particle, there is one electron more than in chlorine atom and the particle as a whole has a negative charge - minus one.

Now we go back to the lab.
Now you know that inside table salt we have ions - sodium plus and chloride minus.
These ions are attracted to each other. And to return to our question - why does the solution of sodium chloride conduct an electric current?

Remember: water consists of uncharged particles, H2O molecules, but table salt consists of charged ions.
Charged particles help electrons pass through the solution, so an aqueous solution of salt conducts electricity, but pure water does not.

What is fluorine minus?
The fluorine atom, which gains an electron, becomes an ion with a minus one charge.
Teacher's notes
Keywords
atoms, ions, charged particles, electrons, anions, cations
Students will
- Learn that there are substances that consist not of atoms, but of ions
- Find out how ions are formed
- See that the size of an atom and its ion are different
- Learn that ions can be positive or negative
Hands-on activities
Before VR
The aim is to show students that dissolving table salt in water produces charged particles able to complete an electrical circuit.
Demonstrate that pure water doesn't conduct electricity. Add a pinch of table salt and note that the solution starts to conduct electricity. (Make a 10:1 consequential dilution to see when the solution stops conducting electricity). N.B. Tap water contains ions and will conduct electricity; distilled water is best for this experiment.
History and sources of knowledge
- Faraday’s discovery of unknown “species” that travelled from one electrode to the other in aqueous media. He thought that these species were generated by electrical current.
- Dissociation theory by Svante Arrhenius (Nobel Prize 1903). Ions form when crystalline substances are dissolved in water.
Topics to discuss
- Why is a sodium atom much bigger than a sodium ion?
- Why is a chloride atom smaller than a chloride ion?
- Substances are electrically neutral. There are no charged substances, but there are charged particles forming electrically neutral substances.
Fun facts and quotes
- Calcium, sodium, and potassium ions are vital for your body to function properly. In addition to other important functions, they greatly affect how your heart works and your thinking process.
- Neon lights glow because of the formation of neon ions inside the lamp. Different noble gases can be used to create different colors (helium: orange, neon: orange-red, argon: violet, krypton: off-white, xenon: blue).
- Ion engines can be used to move a spaceship in space (xenon ions).
- The smallest ion is the hydrogen ion, which is actually just a proton. It's about 2,000 times smaller than a hydrogen atom.
Questions
- How can you form an anion (a negatively-charged ion) from an atom?
- How can you form a cation (a positively-charged ion) from an atom?
- Which is greater for a negatively-charged ion, the number of electrons or the number of protons?
- Which is greater for a positively-charged ion, the number of electrons or the number of protons?
Calculating
- How many protons, electrons, and neutrons does Li+ have?
- How many protons, electrons, and neutrons does F- have?
Quiz
Please see below for the link to a Google form containing a quiz on the material above.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.