The chemical element of francium – structural features and chemical properties
Facts about one of the rarest metals in nature
Francium is an element with the atomic number 87, the atomic mass of its longest-living isotope is 223, and it is a radioactive alkaline metal, with extremely high chemical activity.

The history of the discovery of francium
The metal was discovered in 1939 by Marguerite Perey, an employee of the Parisian Institute of Radium. Evidently out of patriotism, she named the element in honor of her native country. She discovered it while studying the artificially obtained element of Actinium (she noticed an uncharacteristic radioactive luminescence). To be fair, we should note that other researchers may also have worked on creating this element with her at the same time, but the winner is always right, as they say. Main characteristics. This is one of the rarest metals (and chemical elements in general) found in nature.

According to scientists’ calculations, the content of this metal in the earth’s crust is around 340 grams (only Astatine is rarer). This is mainly because of its physical instability – being radioactive, it has a very short half-life (22.3 minutes for the most stable isotope). The only way it can occur naturally is as an intermediary link in the decay of uranium-235 and thorium-232. Thus, all francium that exists naturally is the product of radioactive decay.
How can it be obtained?
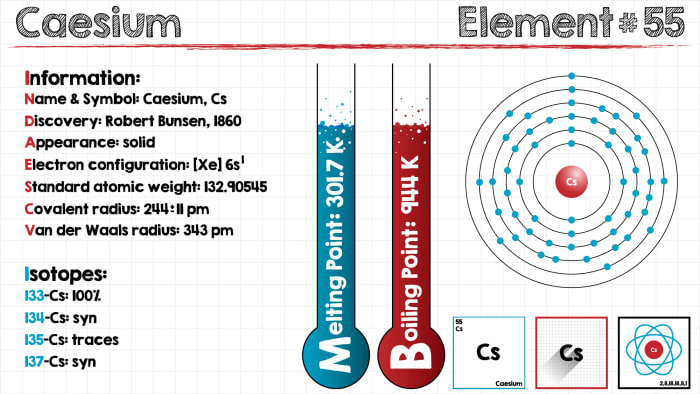
We will look at the only way to obtain the most stable isotope of Francium. This involves a nuclear reaction of gold with oxygen atoms. All other methods (involving radioactive decay) are inappropriate, as the isotopes obtained are extremely unstable, and do not “live” for more than a few minutes. Obviously, it is impossible to create this element in the home, or any of its compounds – and there is really no reason to do so. By its properties, francium is similar to cesium. The relativistic effects of the 6p-shell provide a bond of francium with oxygen in superoxides, for example, the compound FrO2, which is more covalent than superoxides of other elements of this group. Taking into account the maximum low electric negativity of all the elements in existence, Francium is characterized by a high chemical activity. All the physical qualities of this element are only indicated theoretically, as it is impossible to test them in practice because of the short period of “life” of this element (density=1.87 g/cm, melting temperature=27, boiling temperature=677, specific heat of fusion=9.385 kJ/kg). All compounds of this element are soluble in water (an exception is perchlorate salts, chloroplatinate, picrate, cobaltintrite of francium). Francium always crystallizes with substances which include Cesium. We observe its coprecipitation with insoluble cesium salts (perchlorate or silicotungstic cesium). Extraction from francium solutions is carried out by:
- chloroplatinates of cesium and rubidium Cs2PtCl6 and Rb2PtCl6;
- chlorobismutate Cs2BiCl5, chlorostannate Cs2SnCl6 and cesium Cs2SbCl5•2,5H2O;
- free heteropolyacids – silicotungstic and phosphotungstic.
Here you can see dozen interesting experiments with other metals.
What practical significance does this element have?
Despite its uniqueness, Francium does not yet have any practical application. It is not used in industry or in any technology. This is because of the extremely brief period of its half-life. There is data that francium chloride could be used for diagnosis of cancerous growths, but because of the considerable cost of this compound, this method has not been put into systematic use. Essentially, cesium has the same properties.
So this effect of francium is also not in demand – its cost is equivalent to a ton of platinum or gold. According to the predictions of leading specialists, this element will always have pure educational value, and nothing more.