Features and significance of hydration in chemistry
The nature of the process and its main features

Hydration in chemistry has significance for many processes. Hydration in chemistry is the attachment of water molecules to the molecule of another substance, if we’re talking in general terms. It is one of the main topics of study, as other topics in chemistry are based on this knowledge.
The main reason for this is that many reactions and processes take place with water. In order to get a detailed understanding of hydration, we should not only understand the nature of the process, but also its significance.
The nature of the process
In examining the question “what is hydration?”, we may single out a general concept – it is the joining of particles of a substance dissolved in water with water molecules. Solvation is also classified as a type of hydration.
The main difference of hydration from hydrolysis is that this phenomenon does not lead to the destruction of the structure of a water molecule, unlike hydrolysis.
As a result of the process, hydrates form – compounds which have a composition that is permanent or variable.
Crystal hydrates are often salts. They are characterized by the following interactions:
- donor-acceptor;
- dipole-dipole;
- ion-dipole.
If a solid substance is dissolved in water, the crystal lattice disintegrates, and the molecules or ions subsequently go through the solvation process.
As a result of hydration, acids, alkalis or amphoteric compounds may form. Hydration is possible when the molecules of a substance which have excess energy are present in water. In this case they can attach to a water molecule without destroying it.
The most interesting property of crystal hydrates which form as a result of hydration is that depending on the quantity of attached water molecules, substances with different properties may form. Properties that may vary because of this are:
- water vapor pressure
- form of crystal lattice
- solubility
If the same substance is attached to a varying quantities of water molecules, compounds with different characteristics may be obtained.
The reversible process of hydration is dehydration. It is usually applied to solid substances and carried out by:
- evaporation (usually in a vacuum space);
- heating in an open space.
In cases with organic substances for removing water molecules from a substance, hydroscopic agents may be used – for example sulfuric acid.
The significance of hydration
The hydration process has enormous significance for chemical reactions. This is primarily because in many reactions water is present to on extent or another, as water is the main and most popular solvent among all substances. Click here to learn these processes making amazing chemistry experiments.
Not everyone knows that the hydration process is used in industry for the manufacture of important substances:
- acids;
- alcohols;
- aldehydes;
- slaked lime.
Processes of hydration and dehydration constantly take place in the human body. The metabolism process takes place thanks to them. The cells of the organism constantly absorb and release water. At the same time water actively bonds with other substances in the human body.
Examples of reactions
If we are examining hydration, it is important to realize this is a process that can take place with the most varied substances, and also lead to the formation of substances with different properties (salts form most often).
As an example, we may examine the following reactions.
The release of various crystals from a water solution: Na₂SO₄•10H₂O; CuSO₄•5H₂0; ВаС1•2Н₂0. These compounds are called crystal hydrates, and the water in them is water of crystallization. One of these compounds (Na₂SO₄•10H₂O) may easily give up water simply on contact with air.

But CuSO₄•5H₂O may give up water if heated.
Attachment of water molecules:
СаО + Н₂O = Са(ОН)₂ or SO₃ + H₂O = H₂SO₄
These reactions are accompanied by the gradual release of heat. Liquids are usually formed. These compounds are called hydroxides, and the water in them is water of constitution;
Attachment of water molecules to organic compounds
When water bonds with ethylene in the presence of bivalent palladium, acetic aldehyde forms.
Hydration of ions
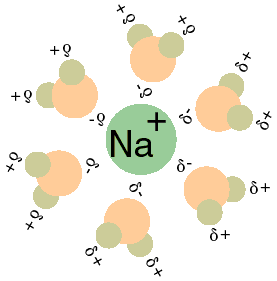
By Taxman - http://bio.winona.edu/berg/ILLUST/Na+H2O.gif, Public Domain, Link
Hydration of sodium-ion
In almost all water solutions each ion is surrounded by a shell of water. These compounds are known as ion hydrates. If you run an electric current through this solution, the ions will not move separately, but rather the ion hydrates will move together. An important property of these compounds is that the hydration of ions decreases as the concentration of the solution increases.
Swelling of colloids
An obvious example is gelatin. These chemical processes have great importance for studying human biology, as they explain the process of muscle contractions, and how swelling forms. In some sources, one may also find the hydration process categorized as a type of hydrolysis. But this is not quite correct, as in the hydrolysis process the molecule of substance breaks down, while in the hydration process the structural features of the water molecule do not change. This issue still remains under discussion, as some scientists are inclined to believe this theory, while others see hydration as a separate independent chemical process.
The study of hydration continues. Scientists often use Monte Carlo calculations, and also molecular dynamics. These methods are promising for expanding our knowledge about the hydration of ions. Combining research methods is considered to be most effective. Modern theories are also based on methods of computational experiments.
These studies are important for industrial discoveries, but also for a detailed study of metabolism processes in the human body.