Iron sparkles
Make a sparkler with calcium nitrate and iron powder!

Safety
-
Put on protective gloves and eyewear. Remove protective gloves before step 7.
-
Conduct the experiment on the plastic tray.
-
Keep a bowl of water nearby during the experiment.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Upon drying, the tube partially unwrapped. May I continue the experiment?
There’s nothing to worry about. The main point is to dry it completely. However, when fixing the paper with forceps, be careful: secure it well yet don’t let it unwrap any further.
The tube is hard to ignite. What to do?
Perhaps, you moistened the paper too well, and it hasn’t yet dried completely. Let the paper dry on the foil for 5 more minutes and then try to ignite it again. Remember to release the tube from the forceps when drying it!
Step-by-step instructions
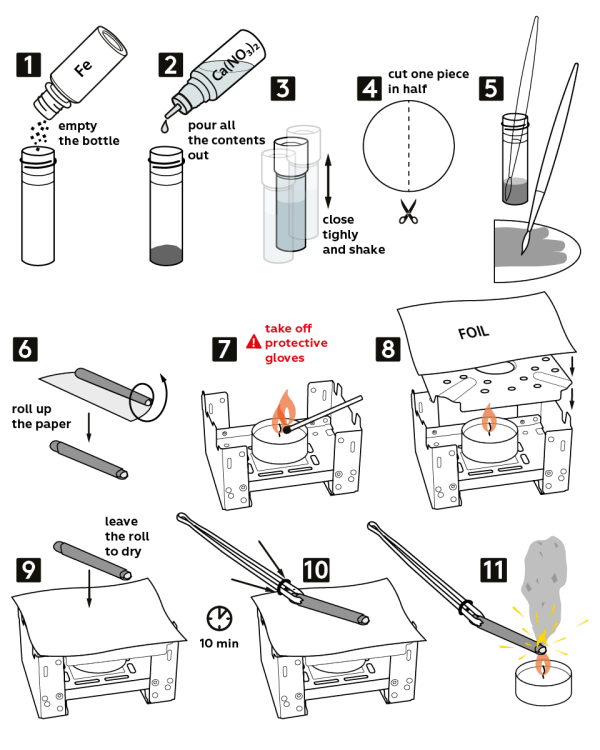
- Pour all the contents from the bottle marked “Fe” (0.4 g of carbonyl iron) into an empty vial.
- Add all of the 3M calcium nitrate Ca(NO3)2 solution (4 mL).
- Tightly close the vial and shake it.
- Take a piece of filter paper and cut it in half.
- Dip the brush into the obtained solution and use it to grease a one-half piece of filter paper.
- Roll a paper tube out of this piece.
- Caution! Remove your protective gloves before proceeding! Place a candle onto the solid fuel stove and light it.
- Cover the stove with the flame diffuser and place a piece of foil on top, as shown.
- Let the paper tube dry on the foil for 10 min.
- Pick up the paper tube with tweezers and secure it in there by moving and adjusting the ring.
- Use the candle to ignite the tube – it will start sparking like a Christmas sparkler!
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Why do we mix iron and the solution of calcium nitrate?
There is no chemical reaction between the solution of calcium nitrate and iron, but we mix these two substances to obtain a uniform mass and evenly spread it over the paper.
Why do we need to dry the paper roll?
Water hinders combustion, so we need to evaporate all of it from the paper.
Why do we add calcium nitrate?
When heated, calcium nitrate decomposes with the formation of oxygen O2. The released oxygen concentrates around the paper roll, so the combustion of paper becomes more vigorous and produces more heat. This heat makes the iron become so hot, that it reacts with the oxygen in the air and produces sparkles.
Decomposition with the formation of oxygen O2 is common for nitrates of several alkaline and alkaline-earth metals, like Na, K, Ca, Ba. Such compounds are called saltpeters. In any nitrate a nitrogen atom is surrounded by three oxygen atoms that pull electrons from the nitrogen. In principle, such a particle is quite stable until additional energy is provided. When solid nitrate is heated, one of the oxygen atoms can leave the molecule by breaking its bond with the nitrogen atom. The released oxygeb atoms form pairs.
Can other metals be used instead of iron?
Such metals as aluminum Al or magnesium Mg would also work in such an experiment and burn with the release of sparkles, but iron is the most common among these. In the video below you can see, how a Bengal light or sparkler, made with the use of aluminum, burns.
But iron does not burn normally. Why does it burn in this experiment?
In this experiment we use iron in the form of a very fine powder. This means, that is easier for the oxygen molecules to get to the iron surface as the surface area is much larger in powders rather than bulk forms. As the powder is fine, more molecules from each granule of the powder can react with oxygen. Solid iron bulk does not burn, because oxygen can only get to the surface of the bulk, but not to it inner parts. As a result, most of the molecules in the bulk remain unreactive.