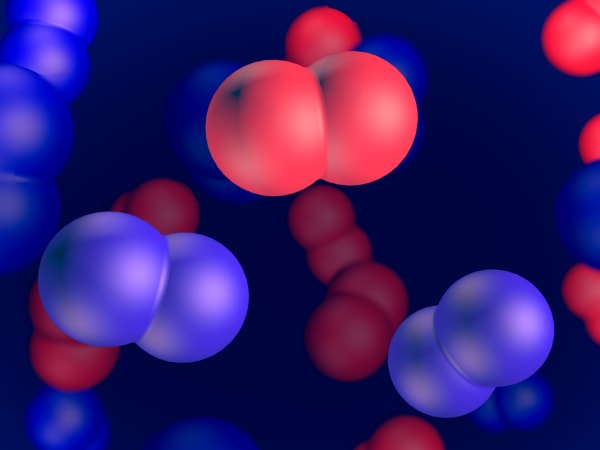
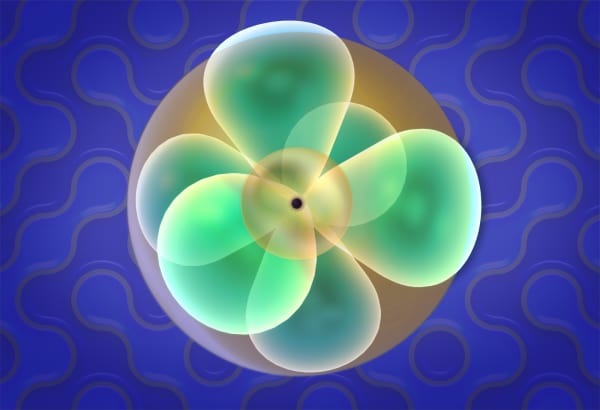
Electron orbitals
Electrons in an atom behave very differently. Students will see that when an electron becomes a part of an atom, it is spread around the nucleus like a cloud. They will also study the shapes of s- and p-orbitals. Later, students will cover the Pauli Exclusion Principle, which states that only two electrons can share the same orbital.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript
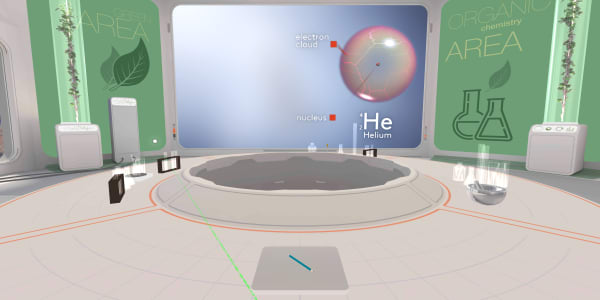
In the previous lesson you saw that an atom consists of a tiny nucleus surrounded by an electron cloud.

But what is this electron cloud? Indeed, it is one of the most important concepts in chemistry.
To understand electron behaviour is to understand why iron rusts, but gold does not,
why we can breathe oxygen, but not helium.
In the previous lesson, we saw electrons in a helium atom. Let's take something with bigger atoms today. Like our pencil.
Let's look inside. Ready to dive?
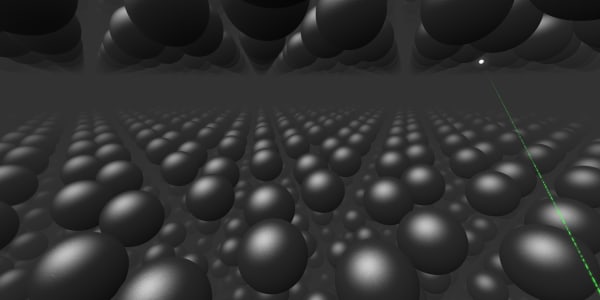
We have to zoom in a billion times to see individual carbon atoms.

Let's choose one of those atoms and get closer.

You can see that in a carbon atom the nucleus is larger than in a helium atom and we see more electrons. And they have strange funny shapes.
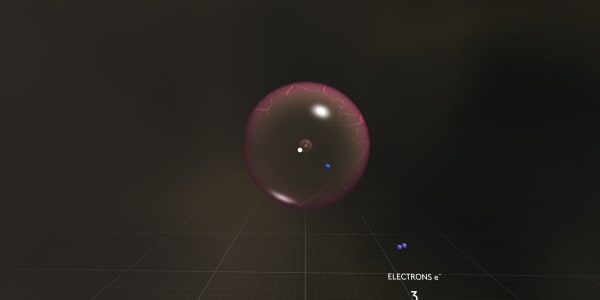
Let's take apart our carbon atom to see what this atom is made of.
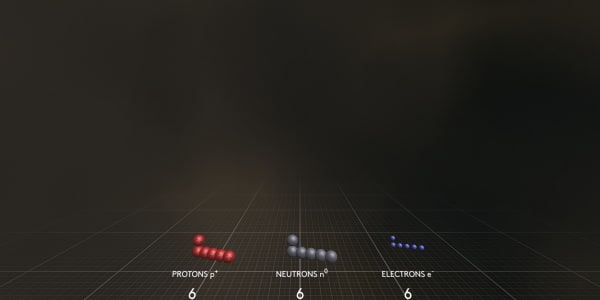
This carbon atom consists of 6 electrons – negatively charged particles; 6 protons – heavy, positively charged particles; and 6 neutrons that have almost the same mass as protons but have no charge.
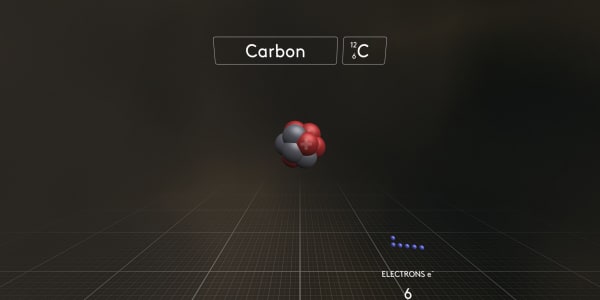
Now let's re-assemble it. First, we will make a carbon atom nucleus with 6 protons and 6 neutrons.
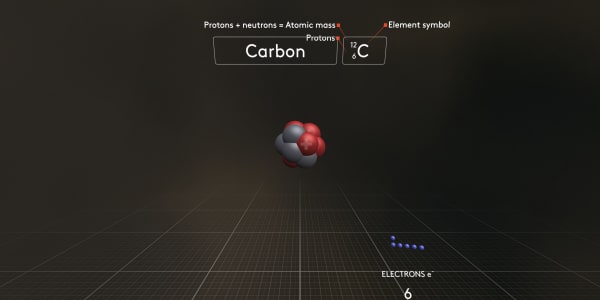
We chemists write a special symbol C_6_12 to show that this carbon atom contains 6 protons and that total atomic mass is 12 which is the number of protons and neutrons combined.
As you already know, electrons are so light that we do not take them into account.
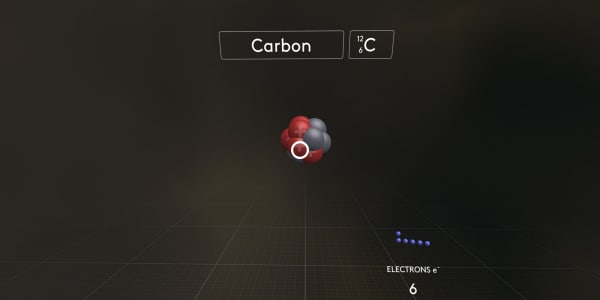
And now the most interesting thing. Let's start to add electrons one by one and see what happens.
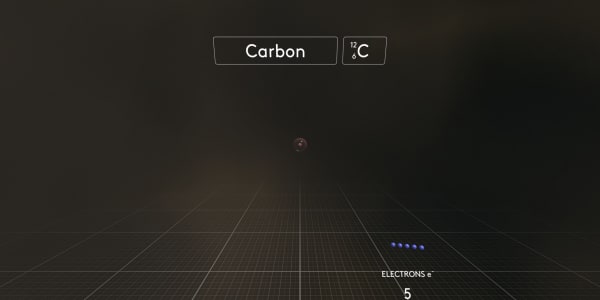
We start with the first electron. A negatively charged electron is attracted to the positively charged nucleus.
But when the electron becomes a part of the atom, it is spread around the nucleus like a cloud, and we call it an orbital.
Does that sound weird? That is how nature works at an atomic level.
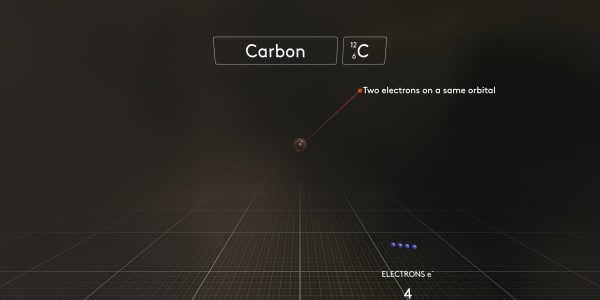
Now let's add the second electron. It will take the same place as the first electron. The first two electrons will share their orbital.
And here is another new fact. Only two electrons can share the same orbital.
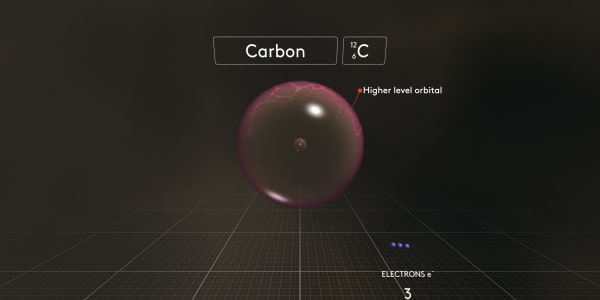
Look what happens when we add the third electron. It cannot go into the orbital where there are already two electrons. So it has to go to the next orbital.
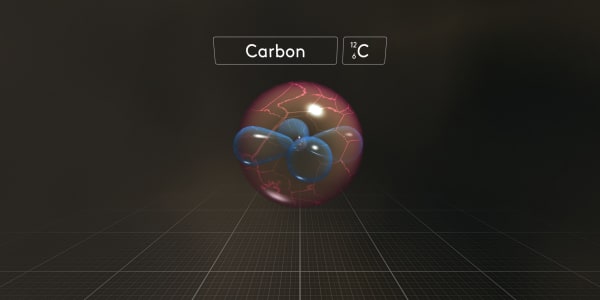
Now just look what happens to the other electrons when we add them one by one.
During the next few lessons you will see why electron orbitals are organized in this way.
This microscale world is very different from the one we know.

Let's go back to our laboratory.
In this lesson you have seen an atom of carbon. In the previous lesson you saw an atom of helium containing two protons, two neutrons and two electrons.
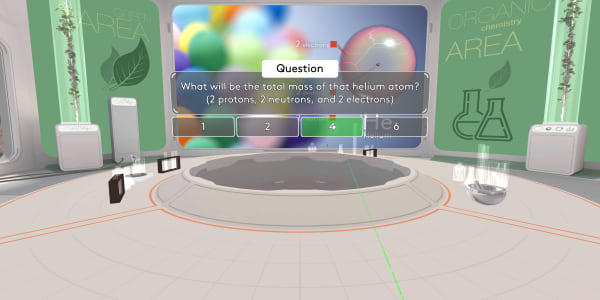
What will be the total mass of that helium atom?
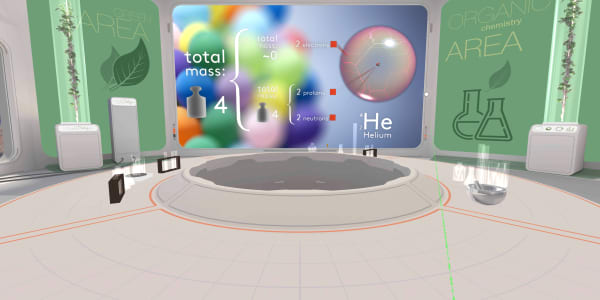
The total mass will be four atomic mass units.
Protons and neutrons have almost the same mass, and we count each of them as one atomic unit.
An electron is more than a thousand times lighter, so we ignore the electrons' mass.
Teacher's notes
Keywords
atoms, electrons, electron cloud, electron orbitals, orbital shape
Common misconceptions
From the Bohr-Rutherford diagram:
- Electrons actively revolve around the nucleus
- Electrons are roughly the same size as protons and neutrons
- The 'distances' from the nucleus to the electrons and between electrons
Students will
- Learn that the nucleus is surrounded by an electron clouds
- Begin exploring orbitals
- Learn that two electrons can share one orbital
- Learn that orbitals have different shapes and sizes
History and sources of knowledge
- History from Rutherford to Bohr and modern theory.
- Quantum theory: description, computational models.
- Scientific approach: the best scientific explanation is based on evidence (observations) and scientific knowledge. A theory should explain observations and make predictions.
Topics to discuss
- How and why did atomic models develop? The scientific approach mandates correcting a hypothesis if it is insufficient to explain new findings (observations, measurements).
- How can we trust theories about things we can't see?
- Emissions and the absorption spectra of elements as a source of information about energy levels.
- Energy level concepts.
- Ladder with a fixed position analogy: when climbing a ladder, you can only step on the rungs' fixed positions, not in between them. Likewise, electrons can only have certain energy levels. In this analogy, the gaps between the ladder's rungs would be much smaller, and smaller still at the top.
- Quantum theory.
Questions
- What’s the difference in shape between s- and p- orbitals? (symmetry description)?
Calculating
- How many electrons do the first 3 noble gases have (with explanation)?
Quiz
Please see below for the link to a Google form containing a quiz on the material above.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.