Blue bottle
Change a solution’s color with a shake of your hand!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the protective underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
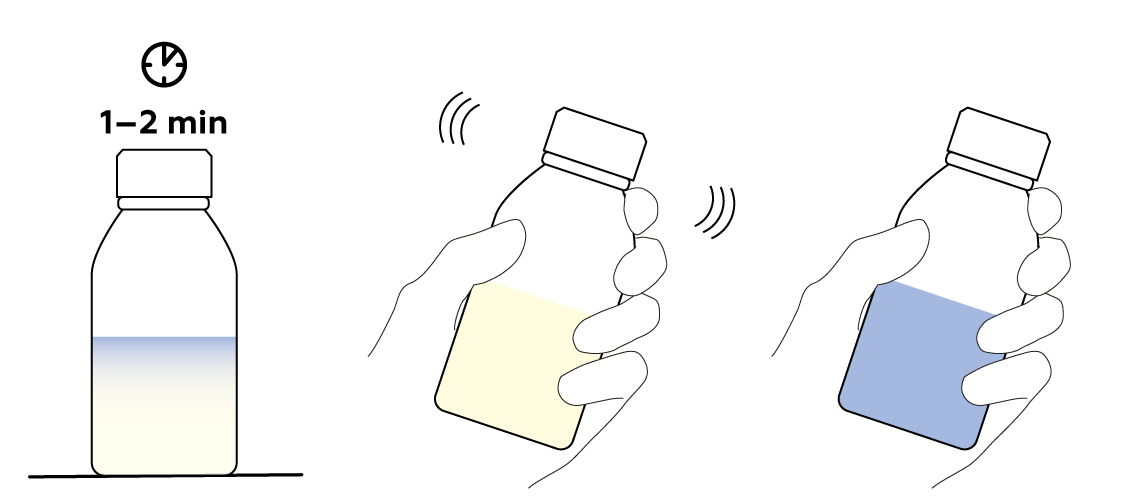
The water can be anywhere from lukewarm to hot. Its temperature only influences the reaction rate. The warmer the water you use, the faster the solution will pale.
No, don’t worry! Continue the experiment.
The liquid will “work” as long as the bottle contains oxygen and lactose supply.
The solution stops turning blue when there is no more oxygen in the bottle to oxidize the methylene blue. To refresh the bottle’s oxygen supply, remove the stopper for a few seconds, then put it snugly back in the bottle. As long as the solution still contains lactose, it should continue changing colors.
Step-by-step instructions
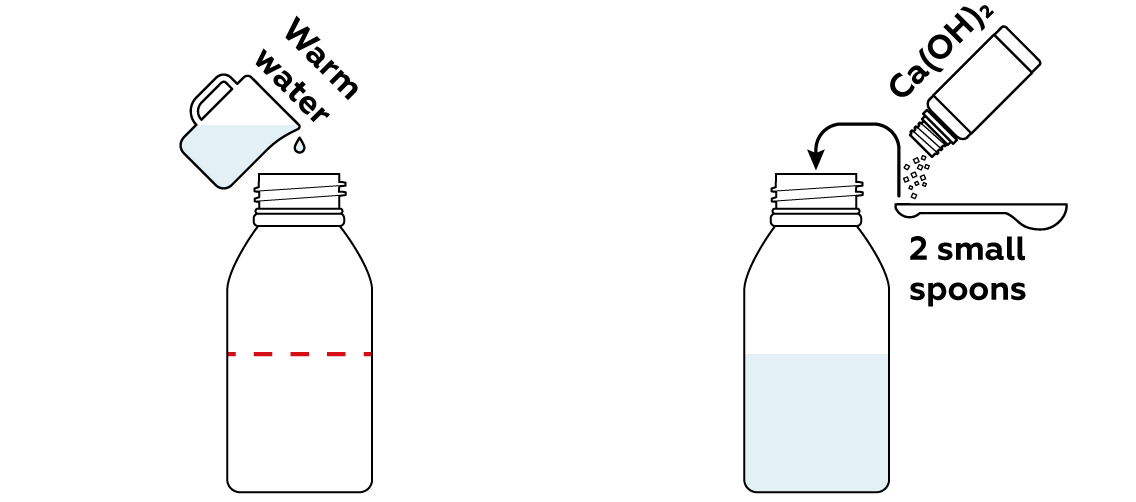
First, use Ca(OH)2 to create a basic solution, i.e. a solution with an excess of OH- particles. The reactions in this experiment will only take place in a basic medium.
Now add some methylene blue and lactose. Methylene blue likes to take electrons from other molecules. And the molecules of lactose, the sugar you find in milk, like to give their electrons away. A perfect match, right?
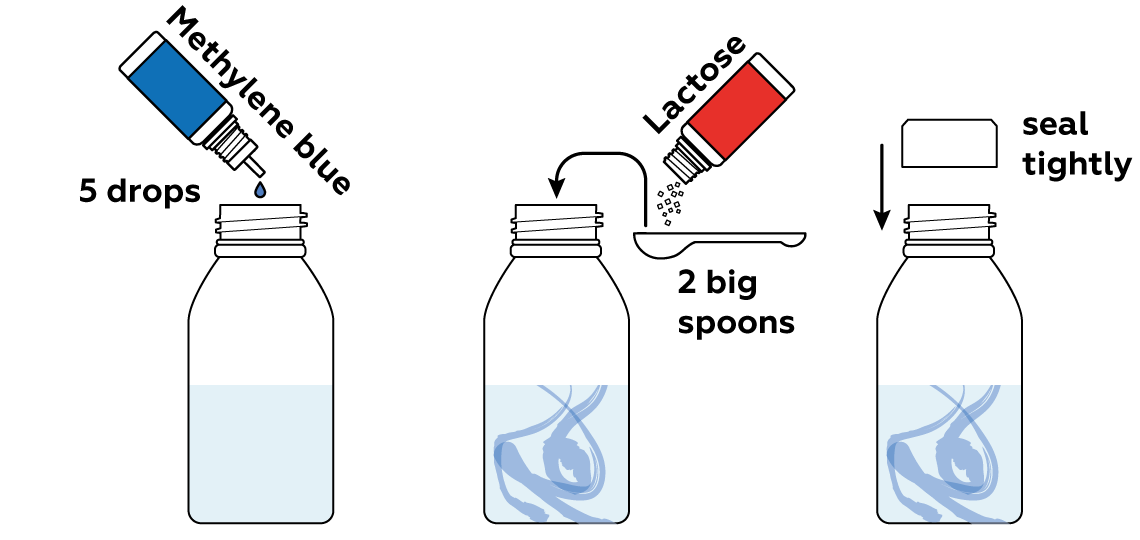
Mix everything thoroughly to help the methylene blue take electrons from the lactose.
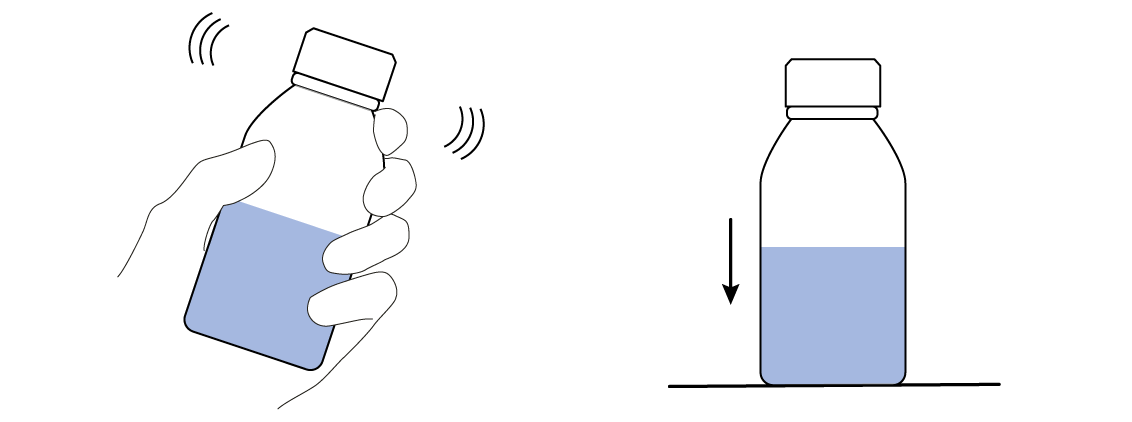
Once methylene blue has its coveted electrons, it becomes colorless. But don't worry—you can easily make it blue again! You just need something to take the electrons from the colorless methylene blue. That something is actually already sealed in your bottle: it's the oxygen O2 in the air that’s taking up about half of the volume of the bottle. Just mix the O2 into the solution by shaking the bottle and watch!
Disposal
Dispose of the reagents and solid waste together with household garbage. Pour solutions down the sink and wash with an excess of water.
Scientific description

going from lactose




That’s interesting!
Why did we add an alkali to the lactose aqueous solution?
By adding calcium hydroxide Ca(OH)2 aqueous solution, we created an alkaline environment. Methylene blue needs an alkaline environment in order to accept electrons from lactose; otherwise, the reaction will not proceed, and the solution will remain blue. You can check this condition by conducting the experiment without Ca(OH)2.
Why is it so important to seal the bottle tightly?
First and foremost, you’ll be able to shake the bottle without sending any liquid flying.
Moreover, in sealing the bottle we are preventing ambient air from entering, and ensuring that the oxygen in the ambient air will not have access to our solution either. This is why the color can only be restored by shaking the bottle (see Why does the solution turn blue again?). The most diligent observers may notice that the blue tint doesn’t disappear completely after the first shake, but remains at the border between the solution and air in the bottle (along the so-called meniscus) and forms a nice blue fringe. The same would happen if the bottle were left open. This is caused by a high concentration of oxygen present in the air above the solution. The oxygen permeates the liquid-gas interface and converts methylene blue to its colored form. However, as the oxygen supply in the bottle is gradually depleted, this border gets increasingly thinner and finally disappears.