Rust protection
Did you know that one metal can sacrifice itself for another?
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Sure! But it must meet certain criteria.
Metals differ from each other in their level of desire to share electrons. Some of them share their electrons reluctantly, while others are more than happy to donate them. Thus, a more generous metal can share its electrons with another one and thereby save it from corrosion. Scientists came up with a list called the electrochemical series, where metals are arranged by their desire to donate electrons:
Cs → Na → Mg → Al → Zn → Fe → Sn → Pb → Cu → Ag
The further a metal is to the left in the series, the more generous it is. Therefore, iron Fe can be protected only by the metals located to its left. Besides magnesium Mg, such metals as aluminum Al and zinc Zn are also generous enough to save iron from its fate. But what about the most active metals like sodium Na and cesium Cs? They are… too good for this purpose! Instead of slowly giving their electrons to both water and another metal, they give their electrons to water extremely quickly, producing a lot of heat and flammable hydrogen gas.
As it is located to the right of iron Fe in the electrochemical series (please see the answer above), copper Cu won’t donate any of its electrons; indeed, it will even take electrons from iron, accelerating its corrosion.
This is indeed the case! That's basically how electric batteries work. You can learn more with our Chemistry & electricity set.
Step-by-step instructions
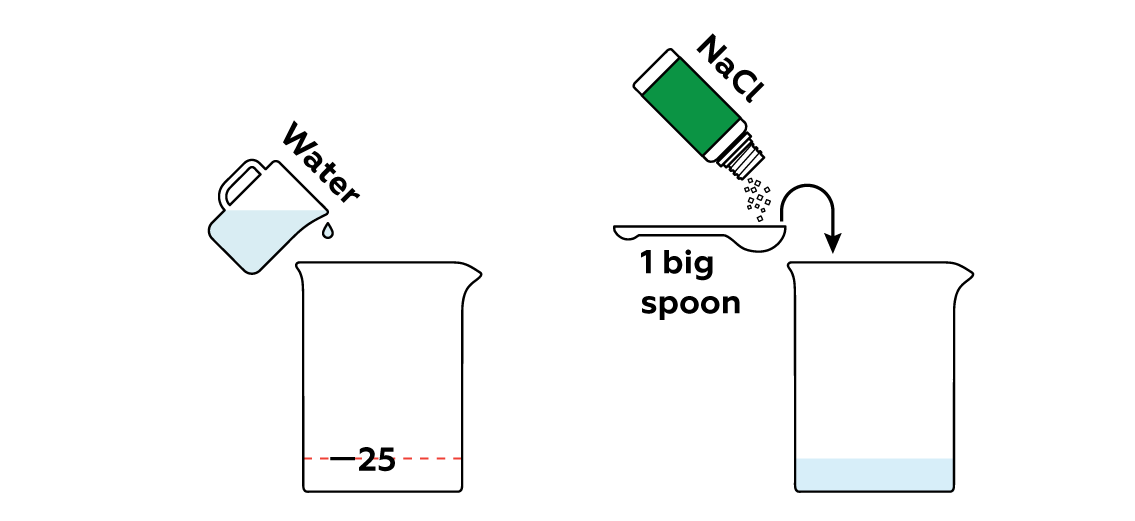
Rust is a special instance of the gradual destruction of metals known as corrosion. Even though this process occurs way faster in salt water (that's why you add NaCl), iron nails still rust too slowly to notice the changes with the naked eye in a short time.
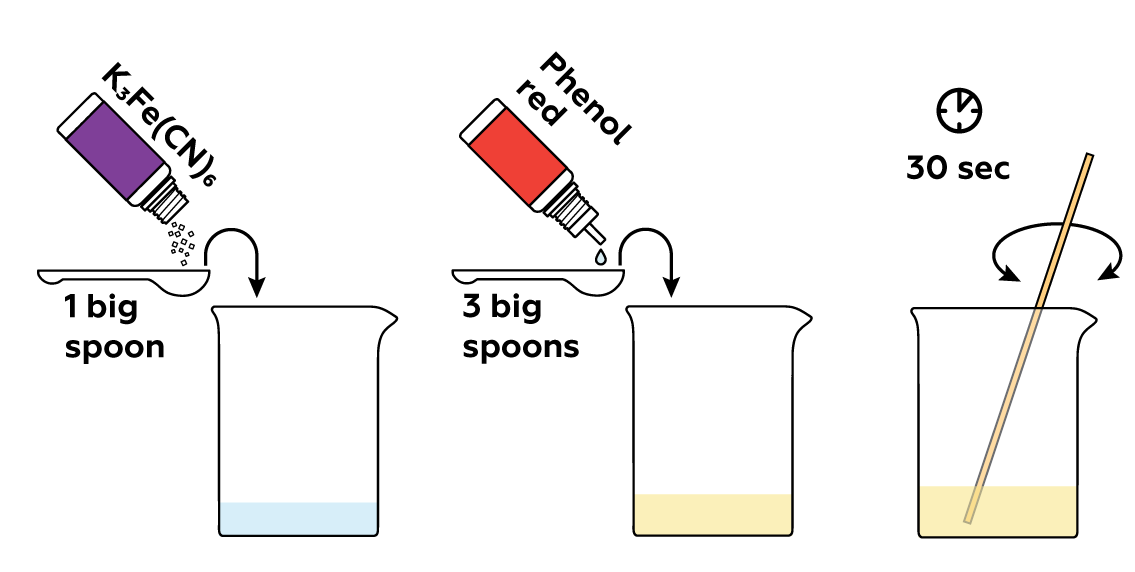
We need some assistance to visualize the process! Potassium hexacyanoferrate(III) K3[Fe(CN)6] and phenol red reveal subtle traces of rust by forming brightly-colored compounds with them.
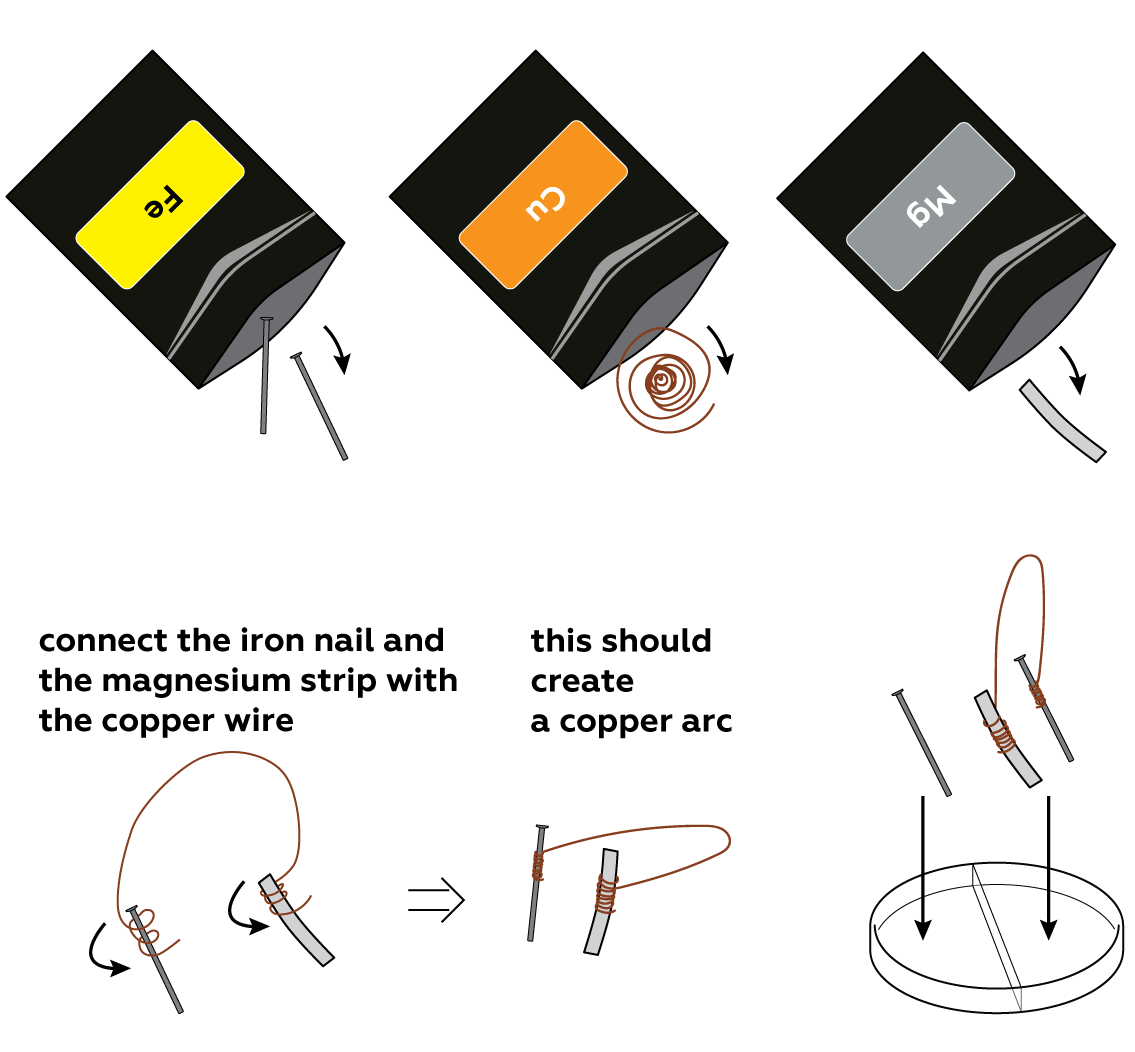
Rust forms when oxygen O2 grabs electrons from iron Fe. The magnanimous iron is eager to donate its electrons—but some metals do it even more easily! Magnesium Mg is one of them. It is so generous that even iron can borrow electrons from it. The copper Cu wire serves as a bridge for the electrons going from the magnesium strip to the iron nail.
On its own, the iron nail rusts in water, and K3[Fe(CN)6] detects this and forms blue algae-like growths on the nail's surface. But in conjunction with the magnesium strip, no blue precipitate forms around the nail and it stays as good as new. How come?
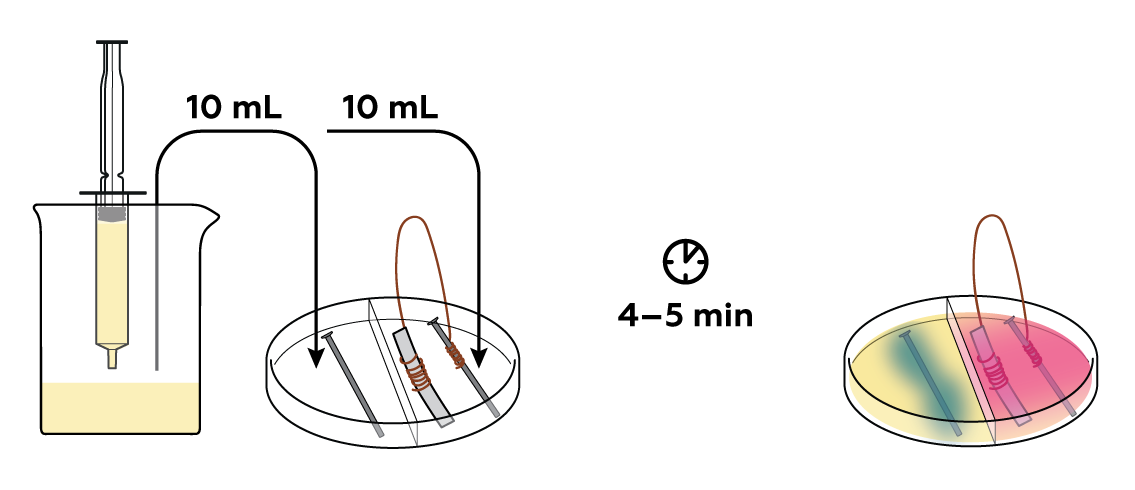
When abandoned one-on-one with oxygen, iron surrenders some electrons. But when bundled with magnesium, iron keeps its metallic form by borrowing electrons from its partner through the wire. Thus, magnesium protects iron from rusting by sacrificing itself. To make sure that this is indeed the case, deprive the iron of spare electrons from magnesium by cutting the wire and see what happens.

Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
Why is the nail covered in blue precipitate? And why does the solution in the second half of the Petri dish turn red?
Since corrosion proceeds rather slowly and the damage to the metal takes a long time to become visible to the naked eye, we resorted to recruiting the help of two substances: potassium hexacyanoferrate(III) K3[Fe(CN)6] and phenol red.
In simple terms, they both help visualize corrosion, but in different ways. The chemical equations of the corrosion of iron Fe and magnesium Mg are similar. When iron Fe corrodes, it is oxidized to Fe2+, while oxygen O2 and water H2O molecules turn into OH− ions:
2Fe − 4e− → 2Fe2+
O2 + 2H2O + 4e− → 4OH−
And when magnesium Mg corrodes, similar products are formed:
2Mg − 4e− → 2Mg2+
O2 + 2H2O + 4e− → 4OH−
Potassium hexacyanoferrate(III) K3[Fe(CN)6] binds with Fe2+ ions as soon as they appear. Together, they form an intensely-colored compound called Prussian blue KFe[Fe(CN)6], which is easily visible even in extremely small quantities:
Fe2+ + K3[Fe(CN)6] → KFe[Fe(CN)6] + 2K+
On the other hand, phenol red changes colors not in the presence of ions of a certain metal – it turns from yellow to red in the presence of another product of any metal’s corrosion – OH− ions. So, when magnesium corrodes, the solution turns red. “But why doesn’t phenol red change colors in the first half of the Petri dish?” – you may ask – “Iron’s oxidation also yields OH− ions!” Interestingly, it does, but the Prussian blue is too intense to see any red. You can easily confirm this by performing the experiment without any K3[Fe(CN)6].
How do we prevent ships from corroding?
You've just protected a small piece of iron with a small piece of magnesium. How would you protect such a huge piece of iron as an ocean liner, then? With a huge piece of magnesium, of course! In fact, that's exactly how it's often done...
Special protectors – bars of aluminum Al, zinc Zn, or magnesium Mg alloys – are attached to the hull of the ship, which is made of an iron Fe alloy. These bars, under the fancy title of "sacrificial anodes," donate electrons to the ship's hull and corrode in its stead, keeping the ship safe and sound. As soon as they deteriorate more than halfway, they are replaced with new ones.
But electrons can move from one piece of metal to another not only due to the difference in their desire to share electrons. The Electricity vs. Iron experiment from our kit shows that the transfer of electrons can also occur with the help of a battery. Thus, another approach to ship protection includes a battery that connects the ship's hull to a metallic bar. The piece of metal connected to the “+” side of the battery corrodes, while the ship's hull, connected to the “–” side of the battery, is securely protected from corrosion.