Flame test
Learn more about metals by burning them!
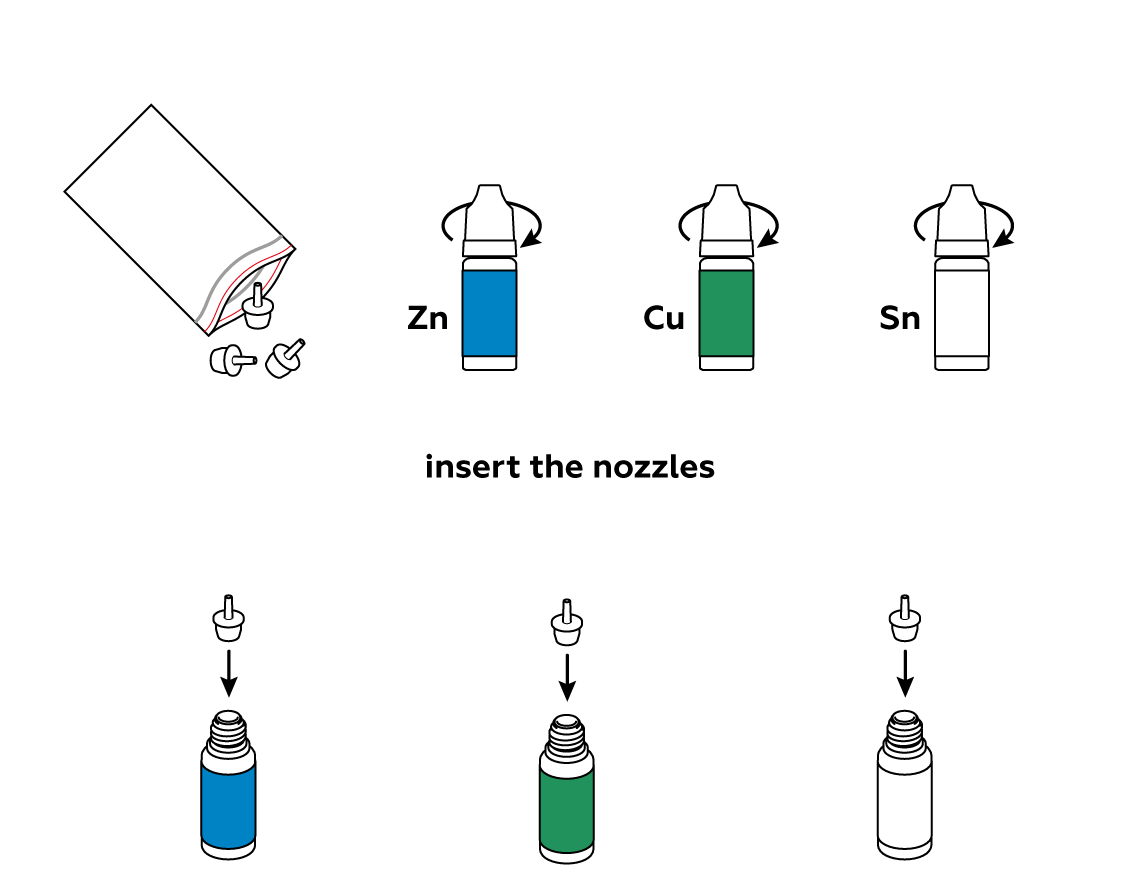
Reagents
Safety
- Put on protective eyewear.
- Conduct the experiment on the plastic tray and in a well-ventilated area.
- Keep a bowl of water nearby during the experiment.
- Keep flammable materials and hair away from flame.
- Place the stove on the cork hot pot stand. Do not touch the stove after the experiment — wait until it cools down.
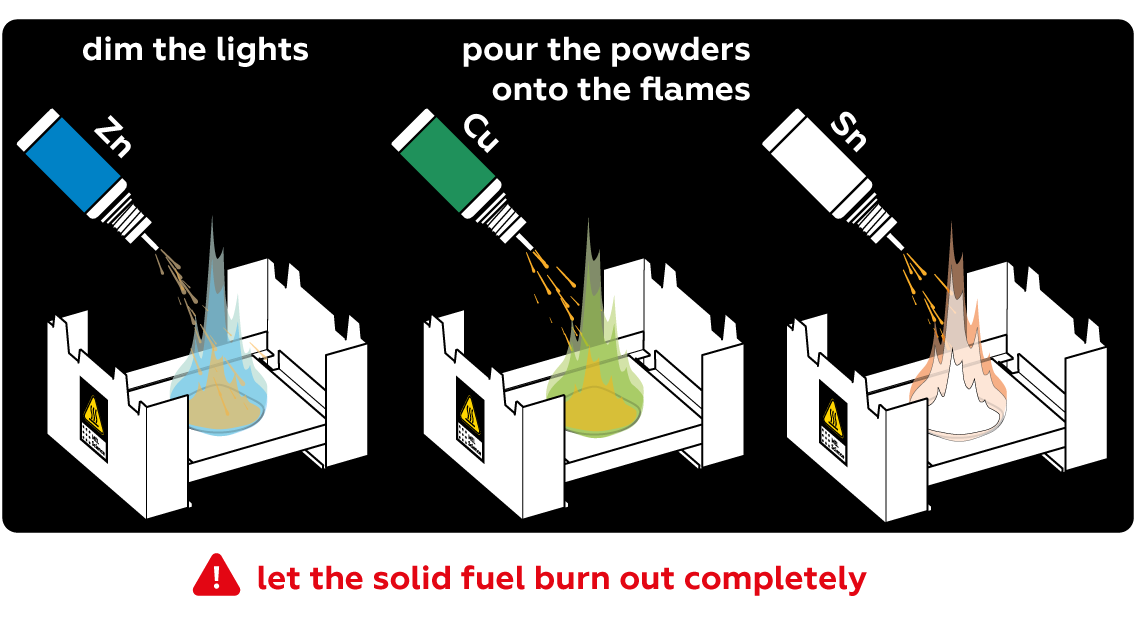
- Do not attempt to extinguish the solid fuel — let it burn down completely.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Switch up the nozzles and try again. The nozzles should fit snugly in the bottles. Of course, you can always ask an adult for help.
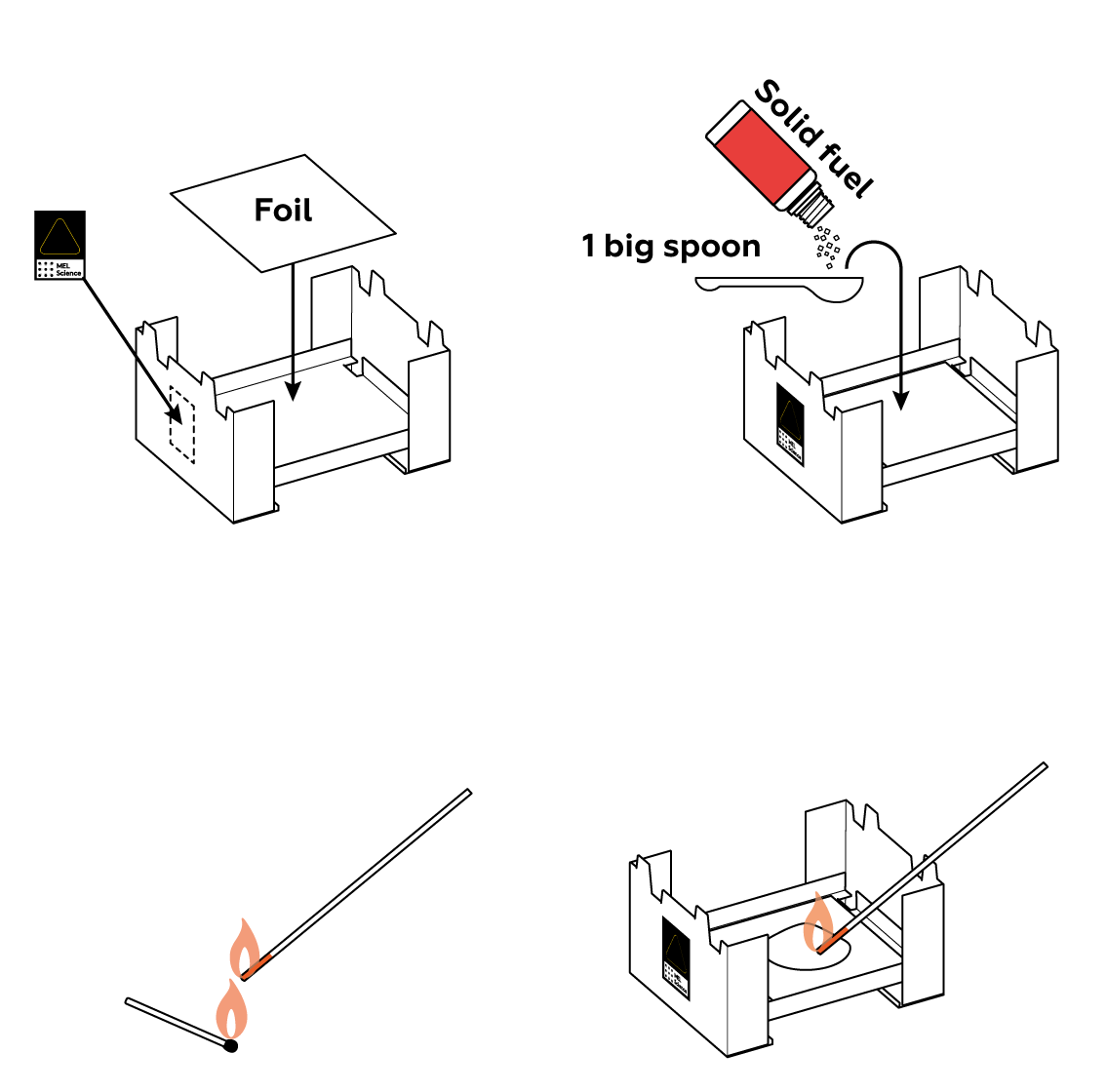
If you look closely, you’ll notice that the foil from the set is much thicker than ordinary foil. Unfortunately, this makes it difficult to replace with foil from another source. We strongly recommend using the foil we provide — it should suffice to repeat the experiment several times.
Use a wooden stick from the set to stir the solid fuel well. This should help break up any lumps and allow you to measure the solid fuel as intended.
Yes, you can continue the experiment.
Point the bottle nozzle toward the flame. Gently tap the bottle to dislodge some powder into the flame. If this doesn’t seem to work, tilt the bottle at a steeper angle and tap slightly harder. You can also shake the bottle like a pepper shaker.
Yes – as a matter of fact, this is a very good idea!
The bottle nozzles are very secure, so it will be difficult to pull them out. If you would like to repeat the experiment in the near future, you can just carefully leave the bottles in an upright position. If this isn’t an option, you can try to find suitable caps from other sets. You can also keep the bottles and nozzles in a resealable plastic bag. Finally, examine the caps from the bottles with powders. If you pull out the round white plugs, these caps can be used for the bottles with nozzles.
Let the solid fuel burn down completely. DO NOT use water to extinguish it. DO NOT blow on the solid fuel or breathe in any fumes. Always work in a well-ventilated area when using solid fuel.
Be sure to wait for the stove to cool down. When the thermosticker turns completely black, you can begin cleaning up.
Step-by-step instructions
We’re used to seeing metals in large chunks or sheets. But they can also be fine powders! While a bigger piece can take a long time to chemically react, a tiny speck can react very quickly. This makes metal powders very useful in chemistry.
You’re about to observe a reaction between your metal powders and the oxygen in the air. In other words, you’re going to burn them! So to start, let’s build a tiny fire.
Pour the powders onto the flames and watch!
Disposal
Dispose of the reagents and solid waste together with household garbage.
Scientific description
When things burn, and metals are no exception, more often than not some rogue particles—ions and atoms 


One interesting thing about this light is that its color is very specific to the kind of atom or ion that emitted it. That's why different metals tint the flame different colors. Zinc makes blue 

That’s interesting!
Colorful flame: the benefits of beauty
For many years, chemists have exploited the capability of some elements to tint flames certain colors. Originally, this property was employed to detect these elements in the compositions of various compounds. Back then, researchers evaluated color and intensity by eye.
Thanks to progress in modern science, this technique developed into photocolorimetry, a method of precise color characterization. Beyond qualitative analysis, special equipment allows for the quantitative characterization of substance composition by flame color. Such data is of great interest to ecologists, geologists, and (of course!) chemists. Unfortunately, this technique only works for certain chemical elements, as many do not tint flames at all—not just visibly, but even in the ultraviolet spectrum range.