Zinc plating
Coat a copper plate with a layer of zinc


Safety
-
Put on protective gloves and eyewear.
-
Conduct the experiment on the tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Why do we wipe the copper plate with hydrochloric acid?
The plate has to be thoroughly cleaned, so that zinc would deposit evenly. Hydrochloric acid effectively cleans off grease and contamination from the drawing surface, which then allows us to create a neat silvery pattern.
I can’t fit the wrapped zinc wire into the tube. Is there an alternative way?
Cut a slit in the tube, enclose the wire, and carefully close the walls of the improvised capsule. Did it work? Perfect! Now, fix the red crocodile clip on the zinc wire (step 4) and follow the instructions to continue the experiment.
The drawing turns out black. What to do?
Most probably, the drawing process was too rushed: if you drew the zinc wire over the plate too fast, zinc atoms didn’t have enough time to form an even, shiny layer. To try again, moisten an absorbent in hydrochloric acid and wipe the plate with it (then wipe it dry with the dry side of the absorbent). Once the drawing surface is ready, try drawing by dragging the wire very slowly.
The plate doesn’t turn silvery. How to fix that?
Perhaps, the copper plate hasn’t been sufficiently dried from hydrochloric acid. Wipe the plate again with the dry side of the absorbent. Add 2–3 drops of ZnSO4 onto the fabric wrapping the wire, and draw by dragging the wire very slowly.
If it doesn’t help, check the connections and their sequence in the circuit. The red crocodile clip should be connected to the red wire of the battery holder, and the black clip—to the black wire.
Examine whether the batteries are inserted in the holder correctly. If their polarity was set correctly, then try replacing the batteries.
Step-by-step instructions
-
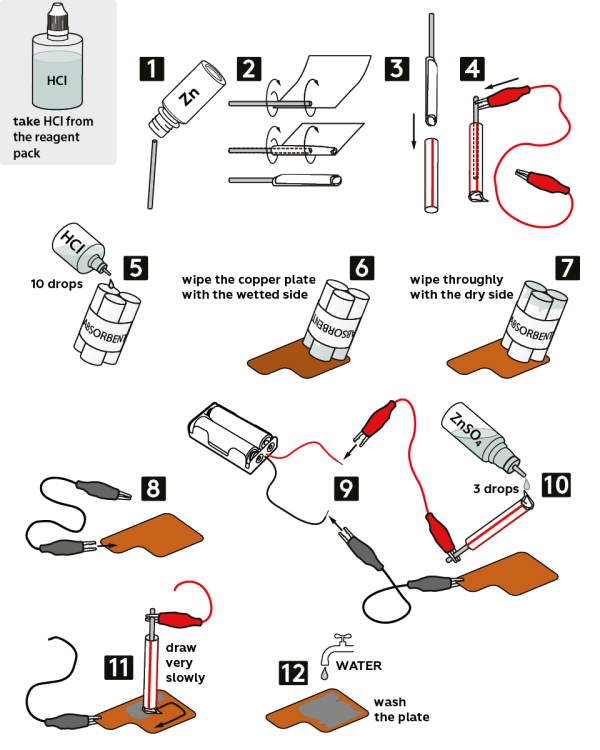
Take a piece of wire from the vial marked “Zn.”
-
Wrap it in a fabric napkin, as shown.
-
Carefully place the wrapped wire into a plastic tube without exposing zinc.
-
Fix the red crocodile clip on the zinc wire.
-
Moisten the absorbent with 10 drops of 2M hydrochloric acid HCl solution.
-
Wipe the copper plate with a moistened side of the absorbent.
-
Next, thoroughly wipe the plate with the dry side of the absorbent.
-
Fix the black crocodile clip on the copper plate “tab.”
-
Connect the loose ends of the crocodile clips to the battery holder: the black clip—to the black wire, and the red clip—to the red wire. Insert 2 AAA batteries into the holder.
-
Moisten the napkin with 0.1M zinc sulfate solution ZnSO4 (3 drops).
-
Now, very slowly draw something with the moistened napkin.
-
Thoroughly rinse the plate with water. You’ve just created a silvery drawing on the copper plate!
Disposal
Dispose of solid waste together with household garbage.
Scientific description
Why does the plate turn silvery?
It happens because zinc, which is a silvery metal, gradually creates a coat on the copper surface during the experiment.
On the atomic scale, most zinc atoms have to travel truly gigantic distances before they “arrive” at the copper surface and form a thin metal layer there! They start from the zinc rod surface, struggle through the cobwebs of the napkin moistened with zinc sulfate solution, finally approach the copper plate, and settle on it.
What is a driving force for such an incredible journey? Even though it may seem strange at first, but the answer is energy from the batteries.
Thanks to the batteries, electrical current flows through our entire system: through the wires, which connect the batteries with the copper plate and the zinc rod, and through the zinc sulfate (ZnSO4) solution inside the napkin that links the plate to the rod.
How exactly does zinc travel from the zinc rod to the copper surface?
How can electricity aid to the transfer of zinc atoms, which are neutral particles and therefore seem to be strangers on this “party” of charged particles?
A chain of events occurring to zinc atoms resembles a situation described in the “Copper coin” experiment from this set. A transfer of zinc from the rod to the plate is realized via so-called electrochemical reactions – transformations of a substance happening due to electrical current flow.
The batteries charge the zinc rod positively. Consequently, some of zinc atoms pass into the solution in form of Zn2+ zinc ions:
Zn0 – 2e– → Zn2+
From here, zinc atoms start their journey in form of ions. With electrical current, positively charged ions move from the zinc rod (which is also positively charged) to the copper plate that, in turn, carries an excess of negative charges. And the napkin material moistened with zinc sulfate solution is no barrier for this transfer. It could filter out sand particles, but against very-very-very tiny zinc ions it is obviously powerless.
Finally, zinc ions arrive at the surface of the copper plate. They would have gladly settled on it via an electrochemical reaction turning them back into zinc atoms:
Zn2+ + 2e– → Zn0
They would have, if only copper oxide (CuO) didn’t stand in their way!
Why should the copper plate be cleaned with hydrochloric acid (HCl) solution?
It is a way to remove a thin oxide layer of CuO from the copper plate. This oxide film does not react itself and prevents zinc ions from approaching copper Cu0.
How can we help zinc ions to achieve their “cherished dream”? The answer is to thoroughly clean the surface of the copper plate using an absorbent moistened with hydrochloric acid (HCl) solution. In water, it completely dissociates into positively charged hydrogen ions (H+) and negatively charged chloride ions (Cl–). Then, hydrogen ions can transform copper oxide into Cu2+ copper ions and water:
CuO + 2H+ → Cu2+ + H2O
Finally, the way for zinc ions is free! Now, they can settle on the copper surface forming a thin zinc layer and rest from their vertiginous journey! They can even cover a whole copper coin making it silvery as shown in this video.
That’s interesting!
Why do they coat ships and cars with zinc?
Galvanizing (zinc plating) is used to protect metal (first of all, steel) works – for instance, parts that mechanisms are made of. Besides all the advantages of iron, it has a significant drawback – iron decays with time because it isn’t air- and moisture-resistant. Other metals (for example, zinc) are also subject to oxidation, but the oxides formed on their surface are dense enough to prevent further metal decay. Unfortunately, in case of iron, the resulting mixture of oxides and hydroxides (basically, rust) is very crumbly, so it cannot protect iron from further oxidation.
In order to protect iron from oxidation, they cover it with layers of paint or coat with other metals. Zinc plating and tinning are the most common methods for such protection. In both cases, iron is coated from the outside with a layer of metal, which is resistant to air and moisture due to forming a dense oxide on the surface. In this video you can see how a rusty metal object becomes silvery and shiny due to zinc plating.
However, there is a conceptual difference between zinc plating and tinning. Should a tinned steel object get even a little scratch exposing steel on the inside, steel starts to rust in that scratched area even faster than if it wasn’t tinned at all. This phenomenon is due to a created galvanic couple on the contact of tin and iron. Here, part of electrons flows to tin, thus, facilitating the transformation of iron atoms on the surface into Fe2+ cations. Luckily, in case of zinc plating, the connection of zinc and iron promotes electron transfer to iron. Then, iron oxidation is slowed down because zinc has to “sacrifice” itself by being oxidized faster than iron.
There are several techniques to coat a steel object with zinc. One on them is called electrolytic zinc plating, and it is essentially the same procedure as in the ”Zinc plating” experiment. In our case, the steel object is represented by a negatively charged electrode (cathode). On this electrode, zinc ions from the solution are reduced. They transform into atoms and settle on the surface. Another well-known technique is to immerse an object in melted zinc (which melting point is around 460 °C) and then cool it in air. The technique is called “hot-dip galvanizing”. You can see how it works in the following video.